Polysialic acids composed of the a(2,8) and/or a(2,9) Neu5Ac units are frequently located at the non-reducing end of biologically active glycoconjugates such as glycoproteins and glycolipids. The use of chemically synthetic oligosaccharides as chemical probes is highly desirable for studying structure-activity relationships.
However, oligosialosides are difficult and challenging synthetic targets.
Now, Takashi Takahashi and colleagues at Tokyo Tech have developed an effective methodology for the synthesis of oligosialic acids by using 5,4-N,O-carbonyl protected sialic acid.
The 5,4-N,O-carbonyl protected sialic acid undergoes a-sialylation in non-coordinating solvents such as dichloromethane to provide a(2,8) disialic acid units in excellent yields and selectivity. The method enabled the first chemical synthesis of a(2,8) tetrasialic acid.
In addition, the Sato and Kitajima group in Nagoya University validated several antibodies using our synthetic oligosialic acid-conjugated proteins. This revealed that the a(2,8) trisialic acid unit, as recognized by mAb.A2B5, resides not only on glycolipids but also on glycoproteins in the developing mouse brain.
In conclusion, this new method for the synthesis of 5,4-N,O-carbonyl protected sialic acid strongly assists any chemical biology research focusing on the role of oligosialic acids.
Reference
- Authors: Hiroshi Tanaka, Yuji Nishiura, and Takashi Takahashi.
- Title of original paper: Stereoselective Synthesis of Oligo-a-(2,8)-Sialic Acids
- Journal, volume, pages and year: Journal of American Chemical Society 128, 7124 (2006).
- Digital Object Identifier (DOI): 10.1021/ja0613613
- Affiliations: Department of Applied Chemistry, Tokyo Institute of Technology.
- Department website: http://www.apc.titech.ac.jp/apc-e.html

- Authors: Emi Inoko, Yuji Nishiura, Hiroshi Tanaka, Takashi Takahashi, Koichi Furukawa, Ken Kitajima, and Chihiro Sato.
- Title of original paper: Developmental stage-dependent expression of a trisialic acid unit on glycoproteins in mouse brain as revealed by mAb.A2B5
- Journal, volume, pages and year: Glycobiology 20, 916 (2010).
- Digital Object Identifier (DOI): 10.1093/glycob/cwq049
- Affiliations: Bioscience and Biotechnology Center, Nagoya University; Graduate School of Medicine, Nagoya University, Department of Applied Chemistry, Tokyo Institute of Technology.
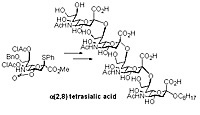
Synthesis of a(2,8) tetrasialic acid.
. Any information published on this site will be valid in relation to Science Tokyo.



