Tokyo Tech News
Tokyo Tech News
Published: November 8, 2013
Since carbon dioxide (CO2) is an abundant carbon source, the catalytic transformation of CO2 into valuable chemicals is one of the most important technologies to achieve a sustainable society.
However, the transformation of CO2 is difficult due to its high stability.
Now, Ken Motokura, Toshihide Baba, and colleagues at Department of Environmental Chemistry and Engineering, Tokyo Institute of Technology have found that Cu-diphosphine complexes can be highly active catalysts for hydrosilylation of CO2 to silyl formate, which is potentially a useful compound for further transformation into fine chemicals.
The catalytic reaction procedure is as follows: a Cu source, diphosphine ligand, hydrosilane, and 1,4-dioxane solvent were placed into a glass reactor equipped with balloon under one atmosphere of CO2, and the resulting mixture was vigorously stirred at 60℃.
The turnover number (TON) and turnover frequency (TOF) of the Cu catalyst reached a maximum of 70000 and 10300 h-1, respectively, with 1,2-bis(diisopropylphosphino) benzene as a ligand.
These TON and TOF values are more than one-order of magnitude higher than those of previously reported catalysts for hydrosilylation of CO2.
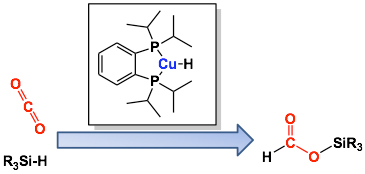
Hydrosilylation of CO2 catalyzed by Cu-diphosphine complex.