1 Polymers
Very large molecules classified into natural and synthetic polymers. Synthetic polymers are composed of repeated specific monomers, and are used in a wide range of fields such as chemicals, automobiles, electronic devices, energy, and biotechnology.
2 Low-molecular compound
Small compounds with low molecular weight, often several 1,000 or less, which are distinguished from biopharmaceuticals.
3 Nucleic acid drug
Drug whose basic structure is a natural and chemically modified nucleotide. Many drugs can be designed for different targets depending on their nucleotide sequences.
4 Biomedicines
Pharmaceuticals made of proteins and nucleic acids, and manufactured, extracted, or semi-synthesized utilizing living things. These differ from low-molecular medicines, and are becoming the trend in pharmaceuticals due to advances in biotechnology.
5 Aptamers
Nucleic acid molecules or peptide molecules that bind to specific target molecules. Usually created from a large random sequence pool and expected to be the molecular alternative to antibodies.
6 Single-chain antibodies
Fusion proteins of the variable regions of the heavy and light chains of immunoglobulins, connected with a short linker peptide. Expected to be an alternative to antibodies.
7 Antibody drug
Made of molecules that bind to the target to deliver the immune function of a living body with an extremely high degree of specificity. Rank highly in the anticancer drug market and have become the mainstream in pharmaceuticals.
8 Glucose
The most important energy source for a living body.
9 Anaerobic metabolism
Metabolism of glucose without oxygen. Energy production efficiency is low, and organic matter is generated.
10 Water-soluble polymer
A synthetic polymer soluble in water.
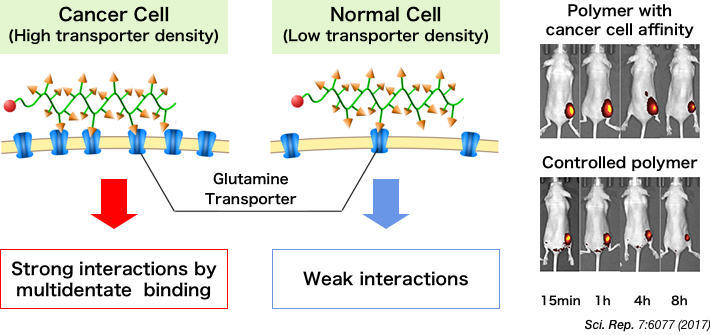
11 Transporter
Exists on the cell surface to transport ions, glucose, and amino acids across the membrane.
12 Antibodies
Responsible for the immune function of the living body, antibodies bind with high specificity to their target, neutralizing and removing pathogens.
13 Cancer antigens
Proteins with which the immune system recognizes cancer cells. HER2, which exists in breast cancer cells, is one of these.
14 Glutamine
A type of amino acid. Cancer cells take in more glutamine and proliferate as more fatty acids, proteins, and nucleic acids are synthesized for the cells to survive.
. Any information published on this site will be valid in relation to Science Tokyo.