Tokyo Tech's Masaaki Fujii and his group have observed atomic site switching in a molecular cluster in real time as part of a UK-Germany-Japan international collaboration. Non-covalent interactions are essential for the functionality of complex molecular systems, such as DNA, proteins, and other supramolecular complexes, which obtain their ultimate properties from the subtle interplay of intermolecular forces.
In particular, intermolecular interactions with OH groups and aromatic rings are well known to be essential for chemical and biological recognition. As an example of such a chemical recognition process, Fujii and his group report the direct observation of hydrophobic-hydrophilic site switching induced by ionization. This switching is initiated in the phenol...argon2 trimer by picosecond resonant photoionization.
When the cation is prepared by photoionization, it is produced in the π-bound geometry of the neutral precursor, with argon binding to the hydrophobic ring site. After seven picoseconds, one of the argon atoms switches from the ring site to the hydrophilic OH site, creating a hydrogen bond. The flight distance of the argon atom is 0.6 nanometers, and the speed of argon is therefore 299 kilometers per hour—the speed of a superexpress train, such as the Eurostar or Japanese Shinkansen. Novel time-resolved picosecond three-color IR spectroscopy allows for monitoring a change in the OH vibrational frequency that indicates the absence and presence of the OH...argon hydrogen bond in real time.
S. Ishiuchi, M. Sakai, Y. Tsuchida, A. Takeda, Y. Kawashima, O. Dopfer, K. Muller-Dethlefs, and M. Fujii
Journal of Chemical Physics 127, p. 114307 (2007).
Department of Organic and Polymeric Materials
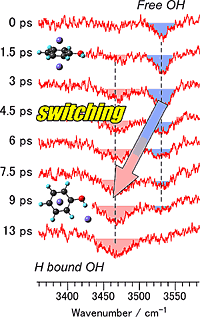
Shown in the figure are picosecond time-resolved IR dip spectra of PhOH+-Ar2 as a function of the delay time after the ionization process. The vibrational wave number of the OH group changes from higher to lower within seven picoseconds. This clearly presents the formation of an OH...argon hydrogen-bond.
. Any information published on this site will be valid in relation to Science Tokyo.



