Yamaguchi and his colleagues have achieved a breakthrough in electrolyte membranes that could broaden the applicability of direct methanol fuel cells (DMFCs). Those fuel cells are strong candidates for new portable power devices. They compare unfavorably in regard to energy density, however, with the lithium-ion batteries now used widely.
Fuel cells are of low weight and small volume because they are open devices, unlike secondary (rechargeable) batteries, which are closed devices. So fuel cells theoretically should exhibit energy density 5 to 10 times greater than that of lithium-ion batteries. The chief problem is methanol crossover: unreacted methanol passes through the electrolyte membrane and reacts directly at the cathode catalyst. That diminishes the energy density of the device greatly.
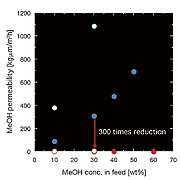
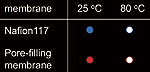
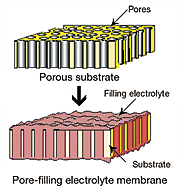
Yamaguchi and his colleagues have addressed the problem of methanol crossover in DMFCs with a highly durable pore-filling electrolyte membrane. They make the membrane from porous polyimide substrate and wholly aromatic hydrocarbon. Their pore-filling electrolyte membrane has enabled them to reduce methanol crossover greatly. The crossover is several hundred times less than with commonly used perfluorinated ionomer membranes, such as Nafion, and proton conductivity is steady. The membrane developed by Yamaguchi and his colleagues is also superior to other membranes in regard to durability.
T. Yamaguchi , Z. Hua, T. Nakazawa, and N. Hara
, Z. Hua, T. Nakazawa, and N. Hara
Advanced Materials 19, pp. 592–596 (2007).
Chemical Resources Laboratory, Tokyo Institute of Technology
Methanol crossover through a Nafion membrane and through the pore-filling electrolyte membrane.
. Any information published on this site will be valid in relation to Science Tokyo.