Orthorhombic molybdenum-vanadium based oxides (orth.-MoVOx) are the most promising selective catalysts for the oxidation of light alkanes. They are particularly useful for producing high yields of acrylic derivatives or acetic acid from propane or ethane.
To make these catalysts even more effective, scientists need to redesign their catalysts based on atomic-scale structural information. However, to date it has not been possible to acquire such information, since orth.-MoVOx do not grow into crystals of the sizes applicable to single crystal X-ray diffraction (XRD).
Now, Masahiro Sadakane and colleagues at the universities of Hokkaido, Hiroshima, Hyogo, Delaware and South Carolina, as well as Tokyo Tech and NIMS, have used XRD to reveal the detailed crystal structure of orth.-MoVOx.
The researchers determined the structure of antimony-containing orth.-MoVOx prepared from {Mo132} polyoxometalate, by using single crystal synchrotron XRD and a high-resolution high-angle annular dark field scanning tunneling electron microscope (HAADF STEM).
The images showed that pentagonal {Mo6O21} building blocks were generated in situ by the disintegration of {Mo132} polyoxometalate. These blocks then incorporated vanadium and antimony while re-assembling into the orth.-MoVOx phase.
The structural information and innovative synthetic procedures established in this research could accelerate the development of highly effective catalytic systems.
Reference
- Authors: Masahiro Sadakane, Keiko Yamagata, Katsunori Kodato, Keisuke Endo, Koshiro Toriumi, Yoshiki Ozawa, Tomoji Ozeki, Takuro Nagai, Yoshio Matsui, Norihito Sakaguchi, William D. Pyrz, Douglas J. Buttrey, Douglas A. Blom, Thomas Vogt and Wataru Ueda.
- Title of original paper: Synthesis of Orthorhombic Mo-V-Sb Oxide Species by Assembly of Pentagonal Mo6O21 Polyoxometalate Building Blocks.
- Journal, volume, pages and year: Angewandte Chemie International Edition 48, 3782-3786 (2009).
- Digital Object Identifier (DOI): 10.1002/anie.200805792
- Affiliations: Catalysis Research Center, Hokkaido University; Graduate School of Material Science, University of Hyogo; Department of Chemistry and Materials Science, Tokyo Institute of Technology; Advanced Electron Microscopy Group, Advanced Nano Characterization Center, National Institute for Materials Science; High Voltage Electron Microscope Laboratory, Center for Advanced Research of Energy Conversion Materials, Hokkaido University; Center for Catalytic Science and Technology, University of Delaware; NanoCenter and Electron Microscopy Center, NanoCenter and Department of Chemistry & Biochemistry University of South Carolina, Graduate School of Engineering, Hiroshima University.
- Department website: http://www.cms.titech.ac.jp/index-e.html

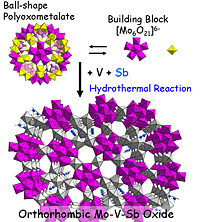
Plausible mechanism of the formation of M-1 phase catalyst.
. Any information published on this site will be valid in relation to Science Tokyo.



