The uranyl(V) ion has one unpaired electron in the 5f orbital (5f1 ), which is the simplest f electronic configuration in actinyl ions (AnO2n+). Hence, studies on the properties of uranyl(V) species should provide data for understanding the chemical properties of AnO2n+ species.
However, to-date studies on the uranyl(V) species have been limited because of its disproportionation to U(IV) and U(VI).
Our group has reported two uranyl(V) complexes existing stably in diemthyl sulfoxide (dmso). After that, Berthet et al. (CEA Saclay), Mazzanti et al. (CEA Grenoble), and Hayton et al. (University California Santa Barbara) isolated stable uranyl(V) complexes in the solid state.
Prismatic crystal of UO2(saldien) was obtained from solution prepared by mixing UO2(NO3)2·6H2O, diethylenetriamine, and salicylaldehyde in ethanol. Electrochemical and spectroelectrochemical properties of UO2(saldien) in dmso were examined using cyclic voltammetry, optical transparent thin layer electrode cell, and so on.
The UO2(saldien) complex can form a stable uranyl(V) complex in dmso, [UO2(saldien)]-, which has characteristic absorption bands at 373, 630, 700, 830, 1390, and 1890 nm. The band at 373 nm is due to the charge transfer from uranyl oxo to U5+, and the other bands are assigned to f-f transitions.
The formation of stable uranyl(V) complexes in solution is predicated by prevention of the formation of an oxo-bridged cation-cation complex. Bulky ligands such as saldien are useful for preventing the formation of the cation-cation complex.
Reference
- Authors: Koichiro Takao, Masayuki Kato, Shinobu Takao, Akira Nagasawa, Gert Bernharrd, Christoph Hennig, and Yasuhisa Ikeda.
- Title of original paper: Molecular Structure asb Elecrochemical Behavior of Uranyl(VI) Complex with Pentadentate Schiff Base Ligand: Prevantion of Uranyl(V) Cation-Cation Interaction by Fully Chelating Equatorial Coordination Sites.
- Journal, volume, pages and year: Inorganic Chemistry 94, 2349 (2010).
Electronic spectra measured at each applied potential for UO2(saldien) in dmso.
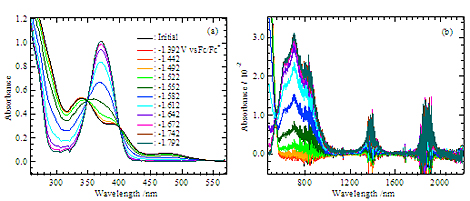
Electronic spectra recorded at the applied potentials in the range from –1.392 to –1.792 V vs. Fc/Fc+ for UVIO2(saldien) (4.20 × 10–3 M) in DMSO containing 0.305 M TBAP. Wavelength regions: (a) 260-570 nm, (b) 480-2200 nm. Noises are due to detectors (photomultiplier and PbS detector) in the spectrophotometer and/or absorption of DMSO in the OTTLE cell. Optical path length : 1.89 × 10–2 cm.
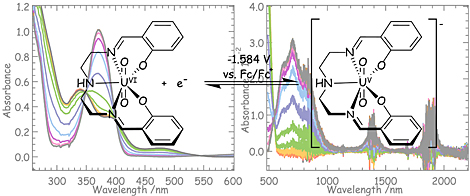
The pentadentate Schiff base ligand fully chelates equatorial coordination sites of UVIO22+, resulting UVIO2(saldien). Since any possibility of cation-cation interaction of UV species was excluded, the corresponding UV complex, [UVO2(saldien)]–, is electrochemically generated as a stable species in DMSO. This UV species also shows characteristic absorption bands due to f-f transitions in its 5f1 configuration and charge-transfer from the axial oxygen to U5+.
. Any information published on this site will be valid in relation to Science Tokyo.




