Tokyo Tech News
Tokyo Tech News
Published: March 31, 2010
Tokyo Tech scientists are creating cheaper, more environmentally friendly cathodes for high-performance lithium ion batteries
Lithium ion batteries are among the most promising power-sources technologies for the future of portable electronic devices, outperforming existing alternatives such as nickel-cadmium or metal hydride cells. They have also been proposed for new generations of electric vehicles and even large-scale energy storage at power plants.
Unfortunately, to date lithium ion batteries have proven problematic for widespread distribution, because they require expensive and sometimes toxic materials. In particular, researchers have been looking for alternative materials for the battery cathodes, which presently contain environmentally hazardous levels of cobalt.
Zhumabay Bakenov and Izumi Taniguchi at Tokyo Tech’s Department of Chemical Engineering are among the scientists tackling this problem. Much of their research has focused on so-called ‘olivine’ structured phosphates, which could be used to build cathodes that are not only low-cost and non-toxic, but also have high energy densities, and are stable under thermal, electrical or chemical changes.
However, olivines have inherent problems, including the existence of a high resistance to the flow of ions and electrons. Researchers have tried various methods to overcome this limitation, including doping the materials with metal ions, and grinding the materials into tiny nanoparticles.
Bakenov and Taniguchi have gone one step further by including carbon when preparing their olivine samples, thereby creating a composite material. The carbon improves the electrical contact between nanoparticles, and prevents the particles from agglomerating into larger chunks which can adversely affect performance.
Recently, the researchers created cathodes from a composite of lithium manganese phosphate with carbon. On placing the cathodes in a battery, they recorded a high discharge capacity, and the samples remained stable at voltages up to 4.9 V and temperatures up to 50 °C.
“The advantages of smaller particle size and carbon composite allowed the performance improvement of the material,” according to the researchers, writing in Electrochemistry Communications1.
In other recent work, Bakenov and Taniguchi made further improvements to their composite material by replacing some of the manganese ions with magnesium. This improved the overall performance of the system. The reason may be that magnesium ions are smaller than manganese, so there is a less significant change in volume when lithium ions enter the cathode. This puts less strain on the system as a whole and improves the insertion and extraction of lithium from the battery cell.
In both of these studies, the researchers made use of two well-established techniques, called spray pyrolysis and wet ball milling. The first involves spraying a precursor solution into an aerosol flow reactor heated to 400 °C. The collected powder is then placed in a milling device and ground up with ethanol and acetylene black for about six hours. The resulting powder is annealed at 500°C for four hours and then finally coated onto aluminum foil to form the cathode.
Bakenov and Taniguchi found that by combining these two techniques they acquired much better-performing samples. As they say in their second paper, published in the Journal of the Electrochemical Society2: “It was shown that a combination of spray pyrolysis and wet ball milling is a versatile method to prepare a high performance cathode for lithium-ion batteries.”
This research was supported by the Development of an Electric Energy Storage System for Grid connection with New Energy Resources under the New Energy and Industrial Technology Development Organization.

Morphology of LiMg0.04Mn0.96PO4/C nanocomposites powders
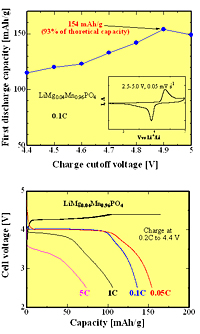
Electrochemical properties of LiMg0.04Mn0.96PO4/C nanocomposites cathode
Reference
Izumi Taniguchi
Graduate School of Science and Engineering Chemical Engineering
Associate Professor