Success in the simple synthesis of organofluorine medicinal/agrochemical intermediates
Published: August 5, 2014
Success in the simple synthesis of organofluorine medicinal/agrochemical intermediates
- Short production process reaction system developed using photoredox catalyst at room temperature -
Summary
At the Chemical Resources Laboratory at the Tokyo Institute of Technology, Assistant Professor Takashi Koike, Professor Munetaka Akita, and Graduate Student Ren Tomita succeeded in directly synthesizing useful trifluoromethyl ketones as medicinal/agrochemical intermediates from easily acquired alkenes (1). This was made a reality by the development of a new reaction system enabling the direct synthesis of alkenes using photocatalysts called photoredox catalysts (2).
A feature of the developed reaction system is its ability to enable the reaction in the mild conditions that constitute room temperature using photocatalysts that act under visible light (LED lamp radiation). Fluoroalkyl groups (3), including trifluoromethyl groups, have a big influence on the chemical/metabolic stability and bond selectivity in pharmaceutical products, and are known to deliver improvements in drug activity, so the new reaction system is expected to see extensive use in the field of medicinal/agrochemical product development in the future.
The results of this research were published in the influential German scientific journal "Angewandte Chemie International Edition," on July 7th and also chosen as the frontispiece.
Explanation of Technical Terms
(1) Alkene:
The general name for a compound having one C=C-binding with unsaturated hydrocarbon. For example, carbonyl compounds (organic compounds having >C=O) such as ketones are obtained by oxidizing alkenes with ozone.
(2) Photoredox catalyst:
A compound such as those shown in the diagram below, including ruthenium complex derivatives with bipyridine ligands and iridium complex derivatives with phenylpyridine. They have an absorption band in the visible light region, and can catalyze one-electron redox reactions using light sources including sunlight, fluorescent lamps, and LED lamps.
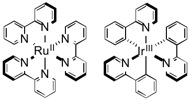
(3) Fluoroalkyl group:
An alkyl group in which a fluorine atom (F) has been substituted for a hydrogen atom (H). Due to the specific nature of the fluorine atom, simple substitution of one hydrogen atom with a fluorine atom creates a large change in the nature of the entire molecule.
Reference
Authors: |
Ren Tomita, Yusuke Yasu, Takashi Koike, and Munetaka Akita |
Journal: |
Angewandte Chemie International Edition |
Title of original paper: |
Combining Photoredox-Catalyzed Trifluoromethylation and Oxidation with DMSO: Facile Synthesis of α-Trifluoromethylated Ketones from Aromatic Alkenes |
DOI: |
|
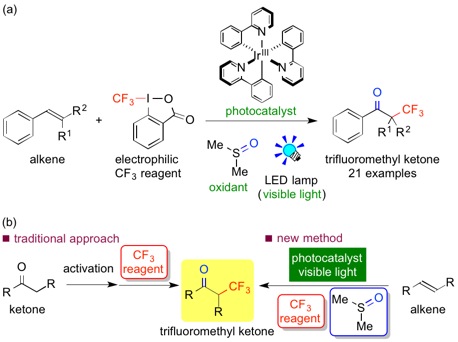
The results of the research (a): A comparison between the traditional approach and new method for oxidative trifluoromethylation of alkenes using photocatalysts (b)
. Any information published on this site will be valid in relation to Science Tokyo.




