High activity of C12A7 electride catalyst clarified
Published: November 4, 2014
Summary
A research group including Professor Hideo Hosono, of the Frontier Research Center and Materials and Structures Laboratory at the Tokyo Institute of Technology, clarified a mechanism that showed high catalyst activity with respect to ammonia synthesis in a catalyst binding Ruthenium to the surface of an electride "C12A7:e-" of a compound 12CaO・7Al2O3(C12A7) made from alumina and lime, which are components in alumina cement. After investigating with ab initio calculations from a model simplifying the reaction, it was clarified that the electron donation of the Ru-binded C12A7:e- exhibited a critical influence on catalyst activity.
It is continuing to become clear that electrides, which are compounds in which electrons serve as anions, have unique physical properties. A typical example is C12A7:e-, which easily donates electrons like an alkali metal, yet remains chemically and thermally stable. A group heading by Professor Hideo Hosono announced in 2012 that binding Ru to the surface of this substance turned it into an excellent catalyst for ammonia synthesis.
The clarification of the current mechanism could be called a result that will lead to the realization of catalysts with even greater efficiency and utility from combinations of carriers with higher electron donation and more generic metals.
The C12A7 electride "C12A7:e-"
A compound with an ionic bond with a naturally electron-electrified skeleton in which the electrons function as anions. C12A7 has a crystal structure composed of a 0.5 nanometer diameter cage-shaped skeleton, and Professor Hosono's group discovered in 2003 that it becomes a stable electride when the baskets are filled with electrons. This property allows it to conduct electricity well like a metal, and it is also known that it exhibits superconductivity at low temperatures.
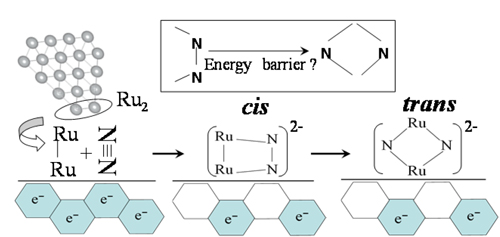
Fig. 1 A model of the reaction
Reference
Authors: |
Navaratnarajah Kuganathan, Hideo Hosono, Alexander L. Shluger, and Peter V. Sushko |
Title of original paper: |
Enhanced N2 Dissociation on Ru-Loaded Inorganic Electride |
Journal: |
J. Am. Chem. Soc. |
DOI: |
|
. Any information published on this site will be valid in relation to Science Tokyo.



