Research group unravels 50 year mystery concerning lead chromate
Published: November 16, 2015
A research group led by Tokyo Institute of Technology in partnership with the Japan Atomic Energy Agency, SPring-8, Waseda University, Chuo University, and Gakushuin University have discovered that the valence distribution of the calcium titanium oxide mineral perovskite is not Pb2+Cr4+O3 which has been purported for 50 years but actually Pb2+0.5Pb4+0.5Cr3+O3. Using synchrotron X-ray scattering and electronic microscopes, the research group's analysis has unraveled a mystery that has eluded researchers for 50 years.
The perovskite compound PbCrO3 has a short-range distribution of Pb2+ and Pb4+ ions and a charge glass state. Pressure induced melting of charge glass and simultaneous charge transfer between Pb and Cr causes a 10% collapse in volume and an insulator to metal transformation. As BiNiO3 (Bisthum nickel oxide) exhibits similar transformations and undergoes great thermal expansion upon chemical modifications, researchers are looking forward to the development of materials for thermal expansion materials based on PbCrO3.
The research group is comprised of Professor Masaki Azuma, Researcher Yunze Yu, and Professor Hajime Hojo from the Tokyo Institute of Technology Materials & Structures Laboratory, Principal Researchers Akane Agui, Tetsu Watanuki, and Akihiko Machida from the Japan Atomic Energy Agency, Principal Researcher Masaichiro Mizumaki from SPring-8, Professor Takashi Mizokawa from Waseda University, Professor Kengo Oka from Chuo University, and Professor Daisuke Mori and Professor Yoshiyuki Inaguma from Gakushuin University. The University of Tokyo, the National Institute of Advanced Industrial Science and Technology, Oak Ridge National Laboratory (ORNL), the Max Planck. Institute, and the Julich Research Centre also participated.
This research was published in the online Journal of the American Chemical Society.
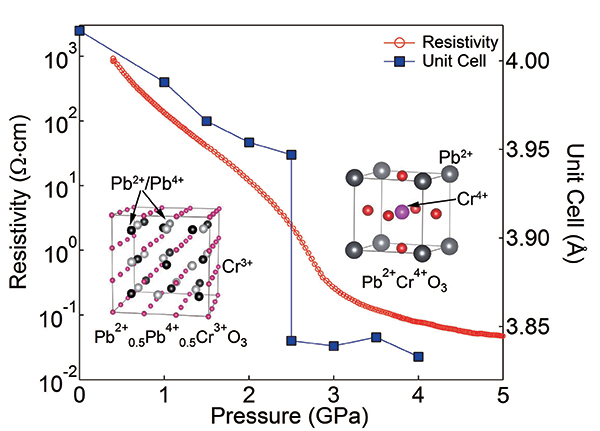
Figure 1.
Pressure evolutions of crystal structure, electrical resistivity and lattice constant of PbCrO3. Abrupt decreases in lattice constant and resistivity owing to the transition from Pb2+0.5Pb4+0.5Cr3+O3 to Pb2+Cr4+O3 are evident.
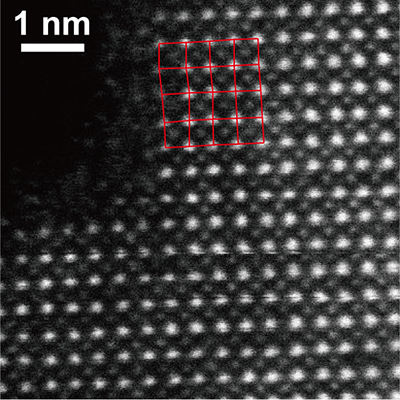
Figure 2. Electron microscope image of PbCrO3 where the lateral shift of Pb positions is evident.
Reference
Authors: |
Runze Yu, Hajime Hojo, Tetsu Watanuki, Masaichiro Mizumaki, Takashi Mizokawa, Kengo Oka, Hyunjeong Kim, Akihiko Machida, Kouji Sakaki, Yumiko Nakamura, Akane Agui, Daisuke Mori, Yoshiyuki Inaguma, Martin Schlipf, Konstantin Rushchanskii, Marjana Ležaić, Masaaki Matsuda, Jie Ma, Stuart Calder, Masahiko Isobe, Yuichi Ikuhara, and Masaki Azuma |
Title of original paper: |
Melting of Pb charge glass and simultaneous Pb-Cr charge transfer in PbCrO3 as the origin of volume collapse |
Journal: |
Journal of the American Chemical Society 137 (2015) |
DOI : |
|
. Any information published on this site will be valid in relation to Science Tokyo.