Researchers from Tokyo Tech and Kyoto University have developed an artificial receptor that can bind sucrose in water with exquisite precision. The achievement represents a leap forward for the development of biosensors, and provides new insights into our perception of sweetness.
Scientists around the world are pursuing the goal of developing synthetic receptors capable of recognizing biologically important molecules. Although many attempts have been made to mimic the way that protein pockets detect sugar dissolved in water with hydrogen bonding interactions, few have succeeded, mainly due to the interfering nature of water molecules. Now, a Japanese team of researchers has proposed a brand new approach.
"Our unique recognition system is based on special interactions — known as CH-π interactions1 — between sucrose and the inner walls of our nanocapsule," says Michito Yoshizawa, who co-designed the study with Masahiro Yamashina at Tokyo Tech. "To our knowledge, nobody has harnessed the interaction for developing this type of recognition system before."
With a diameter of one nanometer (a billionth of a meter), the spherical cavity of the capsule is just the right size to catch the nearly one-nanometer-long and spherical sucrose molecule. Building on the team's previous research on molecular self-assembly, the capsule works by forming a cavity around the sucrose, which then becomes fully surrounded by multiple aromatic panels2 (see Figure 1).
By mixing the capsule, composed of two metal ions and four ligands3, with sucrose in water under mild conditions, the team obtained a sucrose-bound capsule with a high yield. Published in Science Advances, an open-access sister journal of Science, the product structure was confirmed using proton nuclear magnetic resonance and mass spectrometry methods. Yoshizawa adds: "The capsule is easy to produce and handle, and its stability is very high."
In a series of experiments to explore how the capsule would respond to different kinds of sugar, the researchers made three observations: 1) the capsule does not interact with monosaccharides such as glucose and fructose, 2) among common disaccharides (for example, sucrose, lactose, maltose and trehalose), only sucrose was encapsulated, and thus 3) even in mixtures of two disaccharides (in so-called competitive binding experiments), the capsule bound sucrose with a 100% selectivity.
"It's usually very difficult to tell these sugars apart. For example, sucrose, lactose and maltose have the same molecular formula, meaning that they have the same number of hydrogen, oxygen and carbon atoms — only their configuration is different," says Yoshizawa. "Still, our nanocapsule was able to recognize subtle differences and exclusively capture sucrose."
The team also examined how the capsule responded to common artificial sugars: aspartame (known to be around 200 times sweeter than sucrose) and sucralose (around 600 times sweeter than sucrose). The capsule's binding preference was found to be in the order of sucralose, aspartame and sucrose, which exactly mirrors the order in which we perceive levels of sweetness.
This finding could impact the food and chemical industries by helping with the search for even sweeter compounds. If such new compounds can be found and synthesized easily, artificial sweeteners could be produced more cost-effectively than existing methods.
In future, Yoshizawa says that it may be possible to develop "designer nanocapsules" of various shapes and sizes. Ultimately, these capsules could be used for the development of new biosensor technologies in the medical and environmental fields.
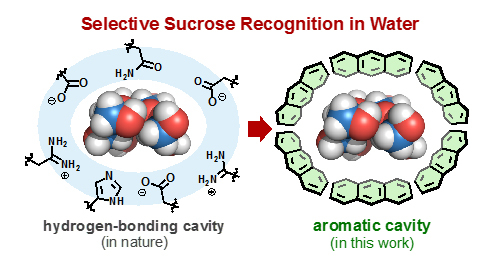
Figure 1. Conceptual cartoon of how a bioreceptor (left) and a Yoshizawa's nanocapsule (right) bind sucrose in their cavities.
1 CH-π interactions
A type of intermolecular interaction found between the surface of aromatic panels and hydrogen atoms bound to a carbon atom (such as a -CH3 group).
2 Aromatic panel
Referring to molecular structures with usually six-member ring(s) composed of carbon and hydrogen atoms.
3 Ligands
Organic molecules that bind to metal ions to form metal-organic hybrid compounds.
Reference
Authors: |
Masahiro Yamashina1, Munetaka Akita1, Taisuke Hasegawa2, Shigehiko Hayashi2 and Michito Yoshizawa1,* |
Title of original paper: |
A Polyaromatic Nanocapsule as a Sucrose Receptor in Water |
Journal: |
Science Advances |
DOI: |
|
Affiliations: |
1Laboratory for Chemistry and Life Science, Institute of Innovative Research
2Department of Chemistry, Graduate School of Science, Kyoto University
|
. Any information published on this site will be valid in relation to Science Tokyo.



