Scientists at Tokyo Tech, led by Prof. Shinji Toyota, showed that nanomolecular structures composed of hydrogen and carbon can take the form of a combined ring and sphere, which looks like the planet Saturn. They analyzed the interactions between the ring and the sphere and explained their origin. This discovery may have implications for the development of new carbon nanomaterials in the future.
- Figure 1.
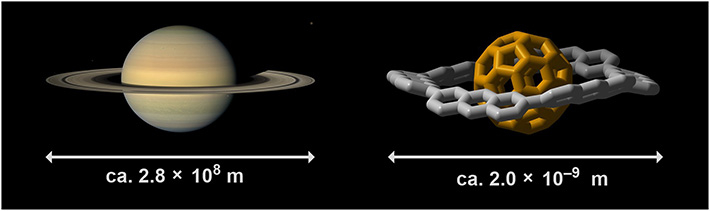
- The planet Saturn (left) and a nano-scale Saturn-type molecule (right). Evidence for the formation of such complexes is scarce, but understanding their interactions could prove very valuable.
Saturn, the second largest planet in our solar system, has characteristic rings that are held in orbit by gravitational forces. Nowadays, nanoscale molecular complexes resembling a miniature version of Saturn are being researched for their potential applications in future materials. A team of researchers at Tokyo Tech in collaboration with Okayama University of Science, Japan, has provided experimental evidence proving the formation of a particular nano-Saturn system.
Nano-Saturn systems are composed of a spherical molecule and a ring-like molecule. Unlike the rings of Saturn, which are held close to the planet by gravity, the ring molecule hosts the spherical molecule inside itself. While previous studies resulted in systems with similar structures, the ring molecules used in those complexes resembled more of a belt or a tire wrapped around a sphere instead of an almost flat disk. This is important because the shape of the molecules used influences the type of bonding shown by the host and the guest molecules, which in turn determines the material properties.
For their nano-scale Saturn-like system, the team used a very well-known type of carbon molecule called fullerene (C60) as the "planet." C60 is made of carbon shapes resembling pentagons and hexagons that bond together to form a hollow sphere, resembling the leather patches in soccer balls (see Fig. 1). Some of the electrons in fullerene are called π-electrons and form an "electron cloud" that can move and bind with other molecules, such as the ones used as the "ring," which also has π-electrons and carbon-hydrogen (CH) groups.
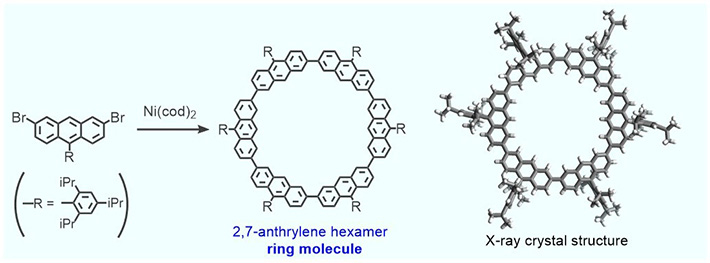
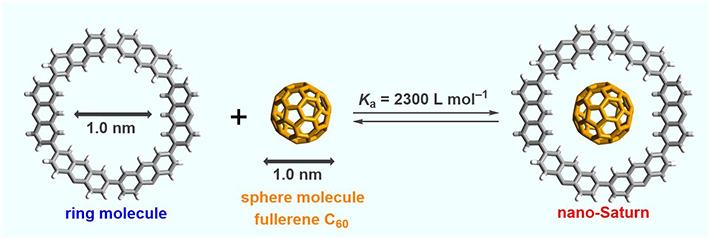
The ring-like molecule used by the team is called cyclic 2,7-anthrylene hexamer, and is produced as shown in Fig. 2. The diameter of the resulting inner cavity is almost identical to the diameter of the fullerene sphere (see Fig. 3), enabling the molecules to show CH···π interactions. Thus, the nano-Saturn system is a potential model for investigating this type of interaction between C60 and arene molecules.
- Figure 2.
- Synthesis of the ring molecule (called 2,7-anthrylene hexamer) and its solid-state structure. Its structure results from the modification of a molecule that the team had studied previously, which did not have the inner cavity where the nano-sphere gets lodged.
- Figure 3.
- Formation of a nano-Saturn complex. The outer substituents of the ring molecule ("R" groups in Fig. 2) are omitted for clarity. The cavity of the ring is slightly larger the diameter of the sphere, which allows it to lodge in and show CH···π interactions.
X-ray analyses, nuclear magnetic resonance (NMR) measurements, and density functional theory (DFT) calculations showed that the nano-Saturn system as a whole is more stable than the individual host and guest molecules not only in the solid state but also in solution. The association constant for the formation of the nano-Saturn system was determined to be 2,300 L mol–1, being 4 times larger than that of previous nano-Saturn system using a flat-shaped outer ring.
Toyota said that the formation of this Saturn-type complex and other host-guest systems should be further studied to develop innovative applications of these complexes for functional materials in the future.
Reference
Authors : |
Yuta Yamamoto1, Eiji Tsurumaki1, Kan Wakamatsu2, and Shinji Toyota1 |
Title of original paper : |
Nano-Saturn: Experimental Evidence of Complex Formation of an Anthracene Cyclic Ring with C60 |
Journal : |
Angewandte Chemie International Edition |
DOI : |
|
Affiliations : |
1 Department of Chemistry and Materials Science, Tokyo Institute of Technology
2 Faculty of Science, Okayama University of Science
|
. Any information published on this site will be valid in relation to Science Tokyo.