Short-lived intermediate phases in solid-gas reactions can be captured with high-speed time-resolved synchrotron X-ray diffraction technique, demonstrate Tokyo Tech researchers. Their observation of the redox reaction pathway for layered perovskite, a high-performance oxygen storage material, enable them to access reaction data within a time window of few hundred milliseconds, highlighting the potential of the technique for optimizing solid-gas reactions.
For the rational design of new material compounds, it is important to understand the mechanisms underlying their synthesis. Analytical techniques such as nuclear magnetic resonance and spectroscopy are usually employed to study such mechanisms in molecular reactions. However, reaction pathways governing the formation of solid-state crystalline compounds remain poorly understood. This is partly due to the extreme temperatures and inhomogeneous reactions observed in solid-state compounds. Further, the presence of numerous atoms in solid crystalline compounds hinders precise analysis. Developing new techniques that can circumvent these challenges is, therefore, necessary.
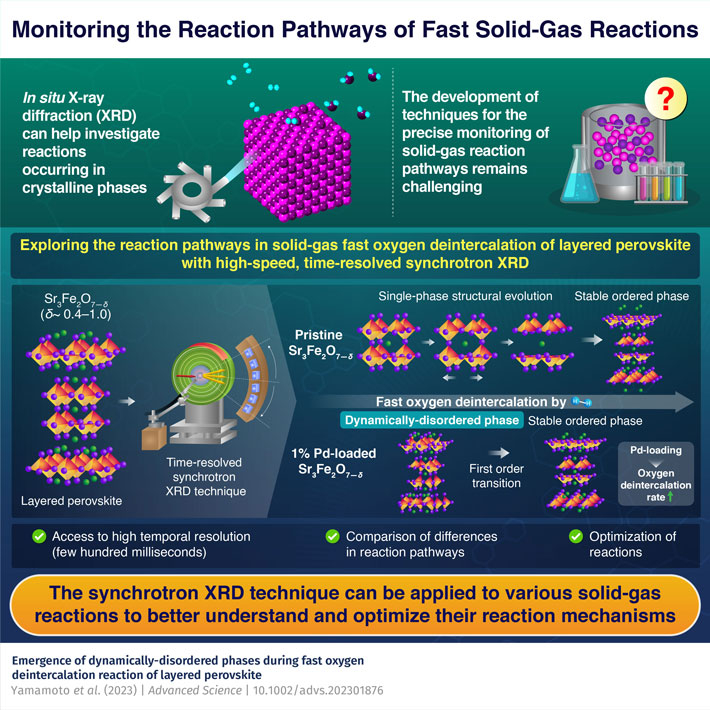
More recently, in-situ synchrotron X-ray diffraction (XRD) techniques have been used for investigating reactions occurring in crystalline phases. Owing to their high speed and temporal resolution, synchrotron XRD measurements provide access to reaction data within extremely short time windows (few hundred milliseconds). This makes the technique promising for capturing data pertaining to short-lived intermediate reaction phases.
Now, a group of researchers from Japan have used such a state-of-the-art synchrotron XRD technique to report the topochemical solid-gas reduction mechanisms in layered perovskite. The study was led by Associate Professor Takafumi Yamamoto from Tokyo Institute of Technology (Tokyo Tech) and published in the journal Advanced Science.
"We used Sr3Fe2O7-δ, a Ruddlesden-Popper type layered perovskite, owing to its efficient oxygen storage ability. Sr3Fe2O7-δ undergoes reversible and fast topochemical redox reactions under O2 and H2 and shows excellent performance as an environmental catalyst material," explains Dr. Yamamoto.
His collaborators had previously observed that doping Sr3Fe2O7-δ with Palladium (Pd) significantly increases the oxygen release rate while decreasing the release temperature. Based on these observations, the team investigated the reaction pathways and structural evolution of this perovskite during the solid-gas reduction.
The team began by preparing a pristine sample and a Pd-loaded sample of Sr3Fe2O7-δ. They then used high-speed synchrotron XRD to monitor them as they underwent fast oxygen deintercalation (reduction).
The analyses revealed that the reduction of pristine Sr3Fe2O7-δ proceeded via thermodynamically stable phases, with pristine Sr3Fe2O7-δ undergoing gradual single-phase structural evolution during its reduction. In contrast, the reduction of Pd-loaded Sr3Fe2O7-δ involved nonequilibrium intermediate phases, a drastically different pathway. It first transformed into a dynamically-disordered phase for a few seconds and then rearranged itself via a first-order transition to reach the final ordered and stable state.
Additionally, Pd metal particles on the Sr3Fe2O7-δ surface significantly accelerated the oxygen deintercalation reaction of Pd-loaded Sr3Fe2O7-δ relative to that of pristine Sr3Fe2O7-δ. Dr. Yamamoto adds, "The change in reaction dynamics following the loading of Sr3Fe2O7-δ with Pd demonstrates that surface treatment can be used to manipulate reaction processes in a crystalline material."
In summary, these findings suggest that the synchrotron XRD technique can be leveraged to study reaction pathways in solid-state compounds as well as identify their rate-determining steps. This, in turn, could help optimize the reaction pathway for the rational design of high-performance functional materials.
. Any information published on this site will be valid in relation to Science Tokyo.