Discovery of the origin of high oxygen permeability in praseodymium nickel oxide-based materials
New design concept of the interstitial ionic conductors by higher d10 dopant and Jahn-Teller effect
A team led by Prof. Masatomo Yashima from the Graduate School of Science and Engineering, Tokyo Institute of Technology and Prof. Tatsumi Ishihara from the International Institute for Carbon-Neutral Energy Research and the Faculty of Engineering of Kyushu University has discovered the origin of the high oxygen permeability in praseodymium nickel oxides containing gallium and copper, Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ.
Ionic conductors with high oxygen permeability and mixed conductors with both high oxygen permeability and high electron conductivity are indispensable in improving the efficiency of fuel cells and oxygen concentrators. Therefore, much effort has been devoted to the development of ionic and mixed conductors. Oxides with the K2NiF4-type structure have recently been identified as mixed conductors with high ionic conductivity.
Prof. Ishihara’s team has previously discovered a Ga3+- and Cu2+-containing praseodymium nickel oxide with the K2NiF4-type structure that exhibits high oxygen permeability. Prof. Yashima’s group has visualized the diffusion pathways of oxide ions at an atomic scale in a similar material, (Pr0.9La0.1)2(Ni0.74Cu0.21Ga0.05)O4+δ. However, the roles of Ga3+ and Cu2+ in the high oxygen permeability of praseodymium nickel oxides and the relationship between interstitial oxygen and oxygen permeability have not been understood well.
The oxygen permeability and oxygen concentration 4±d in three mixed conductors with the K2NiF4-type structure, Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ, Pr2Ni0.75Cu0.25O4+δ, and Sr2Ti0.9Co0.1O4-δ, were measured. Their crystal structures and nuclear and electron densities were analyzed by Rietveld and maximum-entropy methods using the neutron diffraction data obtained by the neutron diffractometer HERMES at the Institute for Materials Research, Tohoku University, located at the research reactor JRR-3M of the Japan Atomic Energy Agency, and using the synchrotron X-ray diffraction data collected by the high-resolution powder diffractometer installed at the BL-4B2 beamline of the Photon Factory, KEK. All the three compositions were confirmed to have the tetragonal K2NiF4-type structure (Fig. 1). Excess interstitial O3 oxygen atoms were observed in Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ and Pr2Ni0.75Cu0.25O4+δ, while Sr2Ti0.9Co0.1O4-δ does not have interstitial O3 atoms but oxygen defects.
The oxygen permeability of Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ is higher than those of conventional mixed conductors, while the oxygen permeability of Sr2Ti0.9Co0.1O4-δ is much lower. In this work the origin of the high oxygen permeability of Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ was found to be (1) a large amount of excess interstitial oxygen atoms formed by higher valence gallium ion Ga3+ and (2) higher mobility of apical oxygen due to longer and weaker copper-apical oxygen bond. At high temperatures between 600 ° and 1000 °C, the nuclear-density distribution of the interstitial O3 oxygen atom connected with that of apical O2 lattice oxygen atom. The connected nuclear densities indicate the oxide-ion diffusion pathway between the O3 and O2 atoms (Fig. 2). The nuclear density around the center of the interstitial O3 atom decreased with increasing temperature, while the minimum nuclear density on the O2-O3 path increased. The oxygen permeation rate increased with the minimum nuclear density, which indicates that the minimum nuclear density is an important parameter that governs the oxide-ion diffusivity.
The present work demonstrates a new concept in the design of interstitial-ion conductors with high oxygen permeability and can lead to the development of new ionic conductors for better solid oxide fuel cells and oxygen concentrators.
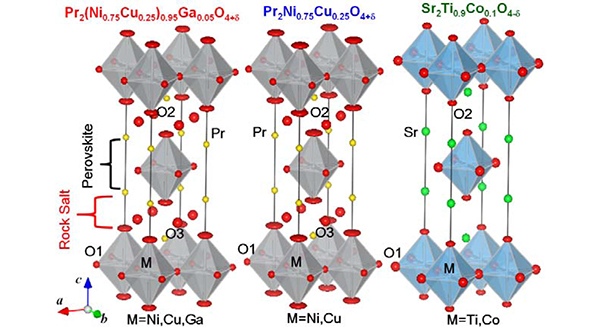
Fig. 1: Refined crystal structures of Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ, Pr2Ni0.75Cu0.25O4+δ, and Sr2Ti0.9Co0.1O4-δ,
which were obtained by the Rietveld analysis of neutron-diffraction data taken at room temperature. Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ and Pr2Ni0.75Cu0.25O4+δ have the excess interstitial O3 atoms.
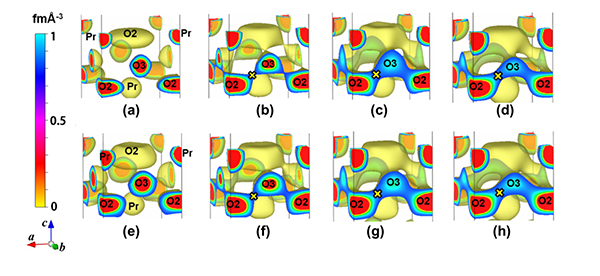
Fig. 2: Temperature dependence of the isosurface of nuclear density in (a–d) Pr2Ni0.75Cu0.25O4+δ and
(e–h) Pr2(Ni0.75Cu0.25)0.95Ga0.05O4+δ. O2 and O3 stand for the apical and interstitial oxygen atoms,
respectively. × denotes the bottleneck with the minimum nuclear density on the O2-O3 diffusion path.
(a) 25 °C , (b) 602 °C, (c) 807 °C, (d) 1011 °C, (e) 20 °C, (f) 605 °C, (g) 810 °C, (h) 1011 °C.
Publication
“Role of Ga3+ and Cu2+ in the High Interstitial Oxide-Ion Diffusivity of Pr2NiO4-based Oxides: Design Concept of Interstitial Ion Conductors through the Higher-Valence d10 Dopant and Jahn-Teller Effect” Chemistry of Materials
Authors:M. Yashima, H. Yamada, S. Nuansaeng and T. Ishihara
Media Contact
Prof. Masatomo Yashima
Graduate School of Science and Engineering, Tokyo Institute of Technology
E-mail: yashima@cms.titech.ac.jp
TEL: +81-3-5734-2225
FAX: +81-3-5734-2225
Prof. Tatsumi Ishihara
International Institute for Carbon-Neutral Energy Research and the Faculty of Engineering of Kyushu University
E-mail: ishihara@cstf.kyushu-u.ac.jp
TEL: +81-92-802-2868
FAX: +81-92-802-2871
High Energy Accelerator Research Organization, Public Relations Office
TEL: +81-29-879-6047
FAX: +81-29-879-6049
E-mail: press@kek.jp
Associate Prof. Kenji Ohyama
Institute for Materials Research, Tohoku University
TEL: +81-22-215-2403
FAX: +81-22-215-2036
E-mail: ohoyama@imr.tohoku.ac.jp
. Any information published on this site will be valid in relation to Science Tokyo.




