Lighting the way to advanced drug design
Published: November 13, 2015
Tokyo Tech researchers create a new way to make important drug motif.
Controlling the shape and arrangement of chemical groups in increasingly complex molecules is incredibly challenging for creators of new drugs. The carbon–carbon double bond is a particularly important building block in many drugs and natural products. This is because the bond is flat and rigid and can secure the position of up to four chemical groups. However, it is difficult to make carbon–carbon double bonds with four chemical groups attached in specific positions.
The search for alternatives
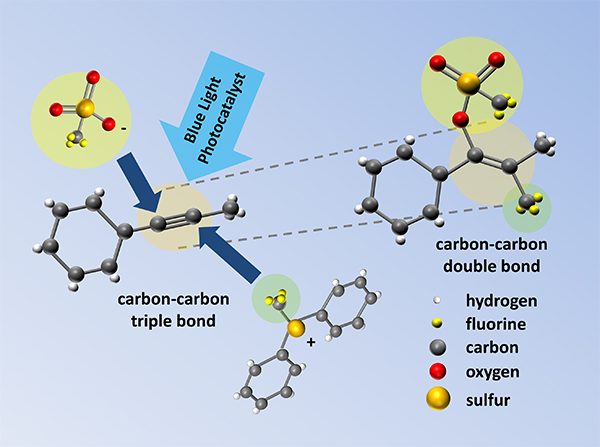
A team led by Takashi Koike and Munetaka Akita at Tokyo Institute of Technology has been investigating ways to improve existing methods for making carbon–carbon double bonds. Starting with carbon–carbon triple bonds, they searched for the right reaction conditions to form double bonds with the addition of useful chemical groups in specific positions.
Figure.
Illustration showing the addition of chemical groups across a carbon–carbon triple bond to achieve a carbon–carbon double bond with four substituent groups.
The right combination
By applying a combination of a metal-based catalyst and blue light during a single chemical reaction, Akita and his group was able to make carbon–carbon double bonds that included a trifluoromethyl group—an important chemical feature of many recent drug target molecules. Most importantly, they were able to achieve control over the arrangement of the different groups around the double bond, and showed that the method could be used for a large range of different starting compounds.
A new route for new drugs
This novel, straightforward approach to making otherwise challenging target molecules will accelerate drug development studies and will also be a useful tool in organic chemistry, allowing new developments in fields such as agricultural chemistry and materials science. The team is now looking at further improving and extending these methods to make other important molecules containing carbon–carbon double bonds.
Reference
Authors: |
Ren Tomita, Takashi Koike and Munetaka Akita |
Title of original paper: |
Photoredox-Catalyzed Stereoselective Conversion of Alkynes into Tetrasubstituted Trifluoromethylated Alkenes |
Journal: |
Angewandte Chemie International Edition |
DOI : |
|
. Any information published on this site will be valid in relation to Science Tokyo.