DNA-based maleimide compound enables titration of reactive cysteinyl thiols in proteins
Published: November 11, 2013
Thiols in protein are often subjected to a variety of oxidative modifications, which play a key role in many cellular processes and are referred to as redox regulation. Therefore, detection of thiol redox states in a specific protein is essential for a deeper understanding of the redox regulation at the molecular level.
Although conventional maleimide compounds can distinguish the redox states of thiols in a given protein, it is difficult to accurately determine the precise number of redox-related thiols due to limitations related to their chemical characteristics.
Here, Satoshi Hara and his colleagues at the Chemical Resource Laboratory of Tokyo Institute of Technology describe the development of a DNA-based maleimide compound (DNA-Mal) and its versatility as a new reagent for investigating redox reagents.
The DNA-Mal was synthesized by conjugating a single stranded DNA to the maleimide group at the 5'-terminus via its aminohexylation.
DNA-Mal with 24 bases can be effectively used to label protein thiols and lead to SDS-PAGE electrophoretic mobility shifts equivalent to ~9 kDa per incorporated DNA-Mal molecule, enabling accurate determination of the number of protein thiols.
The versatility of DNA molecules opens up the possibility of using DNA-Mal for a broader range of cysteine containing proteins.
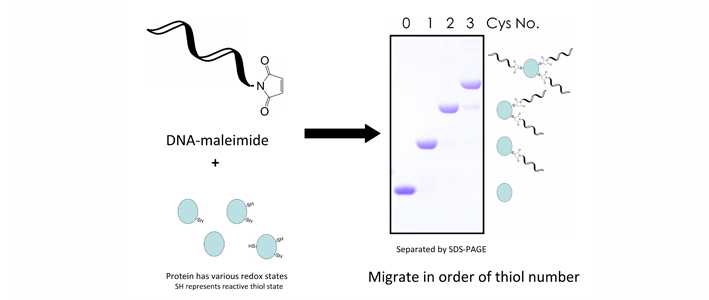
Graphical representation of DNA-Mal. DNA conjugated maleimide group binds to thiols in the protein of interest. The number of bound maleimide is countable from SDS-PAGE migration distance and indicates their redox states.
Reference:
- Authors:Satoshi Hara, Tatsuya Nojima, Kohji Seio, Masasuke Yoshida, Toru Hisabori
- Title of original paper:DNA-maleimide: An improved maleimide compound for electrophoresis-based titration of reactive thiols in a specific protein
- Journal, volume, pages and year:Biochimica et Biophysica Acta 1830, 3077 (2013)
- Digital Object Identifier (DOI):10.1016/j.bbagen.2013.01.012

- Affiliations:Chemical Resource Laboratory of Tokyo Institute of Technology
. Any information published on this site will be valid in relation to Science Tokyo.



