Scientists in Japan have shown that an oxyfluoride is capable of visible light-driven photocatalysis1. The finding opens new doors for designing materials for artificial photosynthesis and solar energy research.
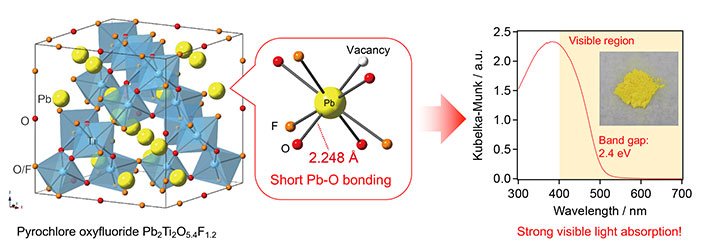
Figure 1. Experiments reveal the strong visible-light absorption of Pb2Ti2O5.4F1.2
The inset (on the right) shows a photograph of Pb2Ti2O5.4F1.2, which is capable of absorbing visible light. This ability is thought to be due to strong interaction between Pb and O, which is enabled by the short Pb-O bond in the pyrochlore lattice.
Over the last decade, research has intensified to develop efficient, manmade photocatalysts that work under visible light — an important target for renewable energy systems.
Now, such efforts have taken a surprising turn, with the discovery of a new photocatalytic material called a pyrochlore2 oxyfluoride (Pb2Ti2O5.4F1.2).
Kazuhiko Maeda of Tokyo Institute of Technology (Tokyo Tech), Kengo Oka of Chuo University and collaborators in Japan have succeeded in demonstrating that Pb2Ti2O5.4F1.2 works as a stable photocatalyst for visible light-driven water splitting and carbon dioxide reduction, with the aid of proper surface modifications.
The new material has an unusually small band gap3
of around 2.4 electron volts (eV), meaning that it can absorb visible light with a wavelength of around 500 nanometers (nm). In general, band gaps bigger than 3 eV are associated with inefficient utilization of sunlight, whereas those smaller than 3 eV are desirable for efficient solar energy conversion.
What's more, the oxyfluoride belongs to a group of compounds that had until now been largely overlooked due to the highest electronegativity4 of fluorine, a property that essentially ruled them out as candidates for visible light-driven photocatalysts.
The new oxyfluoride is "an exceptional case", the researchers say in their study published in the Journal of the American Chemical Society.
Based on structural considerations and theoretical calculations, they conclude that "the origin of the visible light response in Pb2Ti2O5.4F1.2 lies in the unique features specific to the pyrochlore-type structure."
Namely, it is the strong interaction between certain orbitals5 (Pb-6s and O-2p) enabled by short Pb–O bonding in the pyrochlore structure that is thought to give rise to the material's ability to absorb visible light. (See Figure 1.)
One limitation is that the yield of the new photocatalyst currently remains low, at a figure of around 0.01% at 365 nm for hydrogen evolution. The research team is therefore investigating how to boost the yield by modifying Pb2Ti2O5.4F1.2 through refinement of methods for synthesis and surface modification.
The present study arose as a result of collaborations between institutes including Tokyo Tech, Japan Advanced Institute of Science and Technology (JAIST), the National Institute for Materials Science (NIMS), RIKEN, Kyoto University and Chuo University.
The findings are expected to lead to new directions in materials research and future development of heterogeneous photocatalysts under visible light.
1 Visible light-driven photocatalysis:
The process of converting solar to fuel energy using visible-light-absorbing semiconductor materials.
2 Pyrochlore
One of crystal structures represented by a chemical formula of A2B2X6X', where A and B show cations, X and X' show anions. The A and B elements are generally rare-earth or transition metal elements. The presence of two short bonds between A site ion (Pb) and X' site (O) is the characteristic of this structure.
3 Band gap
Refers to the difference in energy of an electron in the valence band and the conduction band, which indicates the conductivity of a material.
4 Electronegativity
A property whereby electrons are held tightly to the nucleus. Fluorine has the highest electronegativity among all elements.
5 Orbitals
The regions where electrons can be calculated to be present within atoms.
Reference
Authors : |
Ryo Kuriki1,2, Tom Ichibha3, Kenta Hongo4,5,6,7, Daling Lu8, Ryo Maezono3,7, Hiroshi Kageyama9, Osamu Ishitani1, Kengo Oka10, and Kazuhiko Maeda1 |
Title of original paper : |
A Stable, Narrow-Gap Oxyfluoride Photocatalyst for Visible-Light Hydrogen Evolution and Carbon Dioxide Reduction |
Journal : |
Journal of the American Chemical Society |
DOI : |
|
Affiliations : |
1Department of Chemistry, School of Science, Tokyo Institute of Technology
2Japan Society for the Promotion of Science
3School of Information Science, JAIST
4Research Center for Advanced Computing Infrastructure, JAIST
5Center for Materials Research by Information Integration, Research and Services Division of Materials Data and Integrated System, National Institute for Materials Science
6PRESTO, Japan Science and Technology Agency
7Computational Engineering Applications Unit, RIKEN
8Suzukakedai Materials Analysis Division, Technical Department, Tokyo Institute of Technology
9Graduate School of Engineering, Kyoto University
10Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University
|
- *
- This article has been updated to correct the title error and a typographical error on May 31.
- *
- This article has been updated to correct a typographical error on May 25.
. Any information published on this site will be valid in relation to Science Tokyo.




