
Issue 15
ProfessorOsamu Ishitani
Department of Chemistry, Graduate School of Science and Engineering
Photochemistry - Chemical reactions by light
Photochemistry refers to a branch of chemistry where reactions are triggered by applying light, not heat, as the energy source. In response to the absorption of light, one compound transforms into another in a reaction based on changes in its atomic bonds.

"The interesting fact is that thermal and photochemical reactions can often derive completely different products from identically structured compounds. Attracted to the mysteries of photochemistry, many global researchers, including myself, have performed countless research activities in the field."
Expressing his fascination at the infinite potential of photochemistry, Professor Osamu Ishitani's eyes shine. He is a researcher with more than three decades of experience in artificial photosynthesis, a promising science often contrasted with solar energy-based biological photosynthesis.
Energy resource shortage is a serious problem for modern society. As a result, there are high expectations for photochemistry in various areas including solar fuel synthesis. Despite now being in the spotlight, photochemistry studies originally started with a completely different focus, according to Ishitani.
"The origins of photochemistry research date back to the late 1800s. Since then, the idea that light would produce different compounds from heat has spawned vigorous attempts to apply it to various compounds and study the results."
An intense adolescent experience
Despite originating out of curiosity, the path to photochemical research swayed toward solar fuel synthesis following the two oil shocks which threatened global society in the 1970s. Amid a sudden, impending sense of crisis, academic, business, and governmental sectors recognized that fossil fuels such as oil and coal would not provide limitless energy.
"My family ran a business selling daily necessities wholesale, and the oil shock hit us hard. Soap and other oil-based products soon ran out and calls from our retailers insisting on supplies made daily life a huge challenge. I vividly recall a tense, sometimes menacing atmosphere."
After graduating from high school, Ishitani chose to study chemistry at Kobe University. He attributes his choice to his love for chemistry, but also the vivid memory of the energy crisis, something that motivated him on the path to photochemistry. One day as a 4th-year undergraduate student, he stumbled across a magazine article on the process of converting light to chemical energy, and the idea grabbed him immediately. At this time the topic of conversion of light energy to chemical energy was becoming increasingly popular.
"The article was written by Professor Zen-ichi Yoshida of Kyoto University concerning the potential to solve the energy crisis with a purely chemical approach. I was very impressed with this, and I knew instantly that this was the only path for me! [laughs]"

Many obstacles lay ahead, but the article had well and truly captured his imagination. From Kobe, Ishitani moved to Osaka University — an institution with a photochemistry lab — and met Doctor Chyongjin Pac, a world photochemistry authority and mentor. Ishitani consolidated and fine-tuned the fundamentals taught by the doctor through activities at the Hahn-Meitner Institute in Germany, the National Research Institute for Pollution and Resources of the Agency of Industrial Science and Technology (presently the National Institute of Advanced Industrial Science and Technology), the University of North Carolina at Chapel Hill, the University of Nottingham, and Saitama University.
At Tokyo Tech, Ishitani currently engages in developing highly efficient photocatalysts.
Photocatalysts and three issues facing humanity
"As concern over the increase in global warming intensifies, the view that fossil fuel consumption must be minimized is becoming increasingly strident. However, I see another key problem: the depletion of carbon-based materials such as organic compounds and synthetic polymers, most of which are petroleum-derived. Knowing how to maintain the stable production of carbon compounds is critical and directly linked to modern life."
Ishitani identifies the challenges for humanity as three major issues: energy, global warming, and carbon-based materials. Aiming to kill three birds with one stone, research into the conversion of carbon dioxide into practical, energy-containing substances such as oil using light energy is underway. This would allow use of solar light energy to obtain ideal carbon cycles which generate energy and carbon resources while helping to reduce CO2 emissions. However, the benefits remain merely theoretical in the absence of efficient photocatalysts.
"To date, the prevailing view has been that highly efficient chemical reduction of CO2 into useful substances is not practical or feasible. Actually, until the late 1970s, the only efficient CO2 reduction method involved electrolysis using currents generated from solar batteries. The fact that spontaneous CO2 reduction occurred when light was applied to photocatalysts was already known. However, efficiency was extremely low as suitable photocatalysts had not yet been tested."
Towards an efficient CO2 reduction mechanism
However, fate conspired to boost Ishitani's CO2 reduction photocatalyst research when he was still an undergraduate. It was the emergence and publication of an astonishing research result in the mid-1980s.
"Professor Jean-Marie Lehn, a French supramolecular chemistry researcher and Nobel Laureate in Chemistry, discovered the ability of a rhenium complex[1] to convert light to chemical energy. Despite its low durability and the fact that nobody had ever used such a rare metal complex as a photocatalyst, its conversion efficiency proved excellent, featuring a 15 percent quantum yield[2]. Simply put, when 100 photons[3] are absorbed into the photocatalyst, 15 carbon dioxide molecules change to form carbon monoxide. This was extraordinary, and I remember feeling goose bumps when reading the paper."
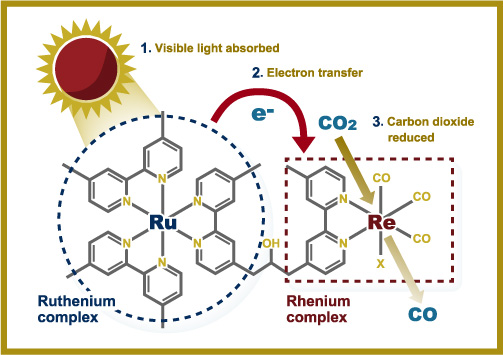
Figure: Rhenium-ruthenium photocatalyst
Ishitani enhanced photocatalyst efficiency by bonding together ruthenium and rhenium complexes, given that the former absorbs visible light more efficiently than the latter.
The encounter eventually prompted Ishitani to start researching photocatalysts based on rhenium complexes in the 1990s. The initial 15 percent quantum yield obtained in Professor Lehn's experiment has been constantly improved by Ishitani, reaching 82 percent in 2013. He has been and remains among the best in the world in this field.
Nevertheless, practical utilization of his research is still unlikely anytime soon, he admits.
"However much lab test results may improve, rhenium remains a rare metal and is not sufficiently prevalent on Earth for its use to be commercialized. I was happy with the positive evaluation of my research, of course, but I couldn't afford to dwell on that. Instead, I had to decipher the mystery of how rhenium complexes could selectively reduce carbon dioxide."
Clarifying the nature and behavior of the factors behind rhenium complex CO2 reduction would facilitate the practical application of Ishitani's research in the future. His insatiable quest has now shifted away from rare metals to the use of other more readily available ones. Accordingly, a new "CREST" project based on Japan Science and Technology Agency (JST) Strategic Basic Research Program initiatives was launched in academic year 2015. The project - led by Assistant Professor Hiroyuki Takeda at the Ishitani-Maeda laboratory - focuses on the application of manganese and iron complexes as photocatalysts.
"This may mark a major step toward commercializing highly efficient CO2 reduction as manganese and iron are far more abundant and affordable."
Tokyo Tech's environment activates global leaders
Ishitani concludes with encouragement for younger generations.

"Define your goals and dreams and act on them. This will enable you to enjoy the laboratory activities you take on in your fourth year. Everyone should find something they want to do, therefore you should visit specialists and listen to them attentively in order to pinpoint what that something is. Be ambitious when choosing your lifetime career."
Finally, he expresses his pride and high expectations for the culture of Tokyo Tech.
"All Tokyo Tech faculty members consider their research to be best in the world. Naturally, I have faith in my team as the world's leaders in photochemical CO2 reduction. Consider this: you too can be number one when you exceed my research. Tokyo Tech makes it all possible. Believe in your potential and jump into our world!"
Explanations of Technical Terms
1. Metal complex
A molecule in which a non-metallic atom or ion, a so-called ligand, bonds to a metal or metal ion.
2. Quantum yield
The ratio to indicate the efficiency level at which photons (light) are absorbed by reacting molecules in photochemical reactions (number of products generated via a photocatalyst ÷ number of photons).
3. Photon
Smallest unit of light.

Osamu Ishitani
Profile
- 2006Professor, Department of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology
- 2002Associate Professor, Department of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology
- 1995Associate Professor, Environmental Control Engineering Course, Graduate School of Science and Engineering, Saitama University
- 1993Visiting Researcher, University of North Carolina at Chapel Hill
- 1992Visiting Researcher, University of Nottingham
- 1991Senior Researcher, National Institute for Resources and Environment (currently National Institute of Advanced Industrial Science and Technology (AIST)) (until 1995)
- 1988Researcher, National Research Institute for Pollution and Resources of the Agency of Industrial Science and Technology (currently AIST)
- 1987Visiting Researcher, Hahn-Meitner Institute
- 1987Doctor of Engineering, Department of Process Engineering, Graduate School of Engineering, Osaka University
- 1982Bachelor of Engineering, Department of Industrial Chemistry, Faculty of Engineering, Kobe University
- 1959Born in Hiroshima
The Special Topics component of the Tokyo Tech Website shines a spotlight on recent developments in research and education, achievements of its community members, and special events and news from the Institute.
Past features can be viewed in the Special Topics Gallery.
. Any information published on this site will be valid in relation to Science Tokyo.










