

Issue 45
Sergei Manzhos
Department of Chemical Science and Engineering, School of Materials and Chemical Technology
We all understand the importance of renewable energy technologies. Devices such as solar panels and fuel cells can help us realise a greener future by adding much needed capacity and flexibility to the energy grid. But much research work is still required to improve these to achieve economically and ecologically sustainable technologies, and these research efforts often remain a mystery to much of the public. Tokyo Tech's School of Materials and Chemical Technology includes many labs working in areas that help develop solutions to society's energy needs. One in particular is Ihara-Manzhos Laboratory, where Associate Professor Sergei Manzhos is busy exploring the world of materials science through theory and modeling. With a strong focus on quantum chemistry and artificial intelligence (AI), he uses these to accelerate the processes of simulation and refinement of materials necessary for the next generation of renewable energy and other technologies.
The origins of a broad approach
Manzhos has studied and researched at several universities around the world, in Canada, Singapore, his country-of-origin Ukraine, and now, for the second time in his career, Japan. While he values his time and experiences at each of the universities in each of the countries he's lived and worked in, he feels the academic scene in Japan suits him well, and so has been with Tokyo Tech since 2021. Though he started out with a degree in physics and radio electronics, Manzhos has gradually expanded into chemistry, mathematics, and computational modeling.

"My background probably sounds a bit broad, doesn't it?", said Manzhos in a recent interview. "But I find all these different things I've done, in all these different places, come in useful to me on a daily basis, as what I do each day can too be very broad. This includes improving modeling methods to make a simulation reflect reality more accurately while using algorithms that run more efficiently. But this is done for specific applications such as development of novel solar or fuel cell materials, and therefore expertise in these fields is also required. And of course, my colleagues and I spend a lot of time working on basic theory, which naturally informs these other things and dictates our direction. One such theoretical area is quantum chemistry."
"The phrase 'quantum chemistry' might sound like something more likely to be uttered in a science fiction movie than in a real science lab," but it's actually a well-established field that uses quantum theory to describe chemical phenomena. The idea comes from the fact that the classical way of thinking about elements, as solid points of mass, charge, and so on, limits what characteristics you can predict about materials made using those chemicals. Using the quantum theory allows researchers to better estimate the characteristics of materials or to evaluate properties that are not accessible with classic approaches such as light absorption, conductance or vibrational spectra. Researchers can search for materials more efficiently using computers rather than traditional bench experiments, making the approach invaluable in R&D.
The use of AI in material science and beyond
"So, we use quantum chemistry to simulate components of things like solar panels, fuel cells, batteries, and other electrical devices. The problem is it is typically computationally intensive to run simulations based on quantum chemistry. Therefore, this dictates a direction for us, to refine existing algorithms and find different methods of implementing quantum chemistry in a computer," said Manzhos. "Common algorithms for such things use a mathematical technique called linear algebra (matrix computations), and we investigate new ways to code these algorithms so that the composite simulations are as accurate as we need them to be, while reducing the number of computational steps required to carry out some operation or another. As linear algebra has wide ranging applications, including things like image analysis, some of our work can find use in fields outside materials science as well. But by far the most impactful thing in the realm of computing that we deal with is, of course, AI."

AI has shaped our daily lives in some obvious, and some more indirect, ways. The nature of AI is often glossed over in the media which can present it as something mysterious and hard to understand. Not that it's easy but seeing it as what it really is deep down, a highly sophisticated way of spotting patterns and generating outputs, data or decisions, makes it easier to see how it can be useful in a field like materials science. Manzhos and his team use AI in a few different ways, for example, to model the electrical grid, to predict materials properties or to enhance simulations. Often, the data he works on is highly dimensional, containing a large number of variables which may also depend on each other, and also many different data types. AI is advantageous for such applications due to its ability to uncover implicit information in large and possibly heterogeneous datasets, something that may be difficult or impossible with traditional techniques.
"Many instances of AI used in modeling don't necessitate a great level of accuracy; for example, let's say you're predicting power consumption by the hour in an energy grid. The smallest number you might be concerned about is something like 10 watts, one LED bulb. That figure might represent 1 percent, maybe a little less, the consumption of a household. So the AI needed for such modeling doesn't need to be so accurate," said Manzhos. "But for some of the applications we work on, including AI for improving quantum chemistry based modeling methods, we might need 100 times the accuracy of that. We often run into the limits of what contemporary AI can achieve, hence the reason we adapt existing algorithms to our needs and also develop novel algorithms that can then be used in other areas as well."
Refining basic processes has far-reaching benefits
Manzhos initially turned to AI as a means to try and solve problems that did not have efficient solutions using classical means to solve them. One such example is a class of functions called interatomic potentials. These are needed to calculate the potential energy of a system of atoms, in other words, how much energy a molecule has that can be converted into another form under some process. An everyday example of where such functions might be useful is when engineers are creating something that might expand or contract with a change in temperature. But as these functions can require long, exhaustive searches for solutions (unless one is willing to settle for simple but not very accurate approximations), which take a lot of computing time and energy, AI can be applied instead. Machine learning is advantageous as it can build a solution without the researchers predetermining its form.
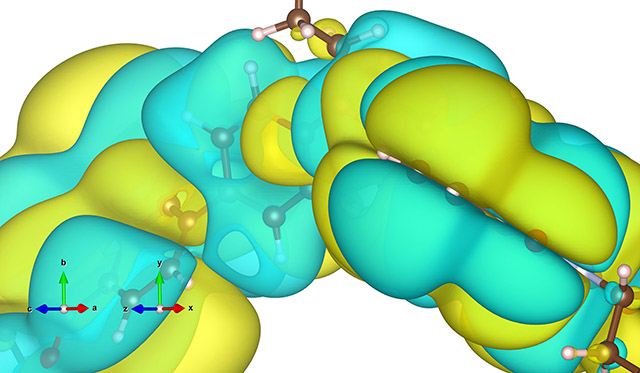
Electron wavefunction distribution in a molecule (shown as isosurface)
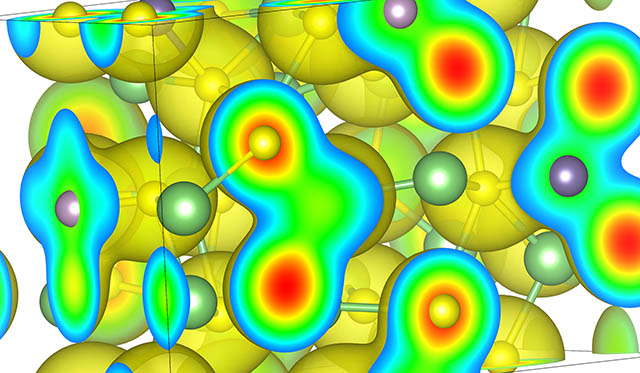
Potential energy landscape (shown as isosurface) in which Li ions move in a solid-state battery electrolyte
This, however, becomes increasingly difficult as the dimensionality of the problem increases and one gets hit by the so-called "curse of dimensionality" reflected in particular in an exponentially growing need for more and more data to build a reliable model. Dr Manzhos and colleagues have been developing novel machine learning algorithms capable of building reliable models even in very high-dimensional spaces and from sparse data. These algorithms are hybrids of neural networks, Gaussian process regressions, and an approach known as HDMR (high dimensional model representation) that is a framework for representing high-dimensional functions with low-dimensional terms.
Some other areas of study Manzhos applies AI to have existing industrial or governmental counterparts, such as the modeling of energy consumption. This is something that energy organizations have to deal with. They can therefore benefit from developments made by his group. Other researchers benefit from the work Manzhos does on improving simulation capabilities at the quantum scale, in particular by making them applicable to more realistic lengthscales. For example, some scientific fields explore expressions of quantum phenomena, including things like protein folding, essential for medical developments. A common method used for some quantum modeling is called 'density functional theory' (DFT) [1]; however it too is computationally expensive. Manzhos explores alternatives to this method, as it is a bottleneck to modeling materials, despite being widely used at present.
Manzhos and colleagues recently proposed a machine learning-based improvement to a DFT alternative called density functional tight binding (DFTB) [2] and used it to compute an interface of Si/SiOx/TiO2, which are important materials in silicon-perovskite tandem solar cells. The team demonstrated that although modeling the thousands of atoms required by the interface would be an unfeasible task for DFT, it was a relatively easy calculation for the DFTB method. This in turn illustrated how their improvement makes the DFTB method applicable to a wider range of materials.
Engineering around the limitations of conventional methods like DFT will allow researchers to model materials in unprecedented detail and potentially without the need for newer, more expensive hardware. This could also improve accessibility to research methods, as more costly alternatives might be out of reach to many around the world.
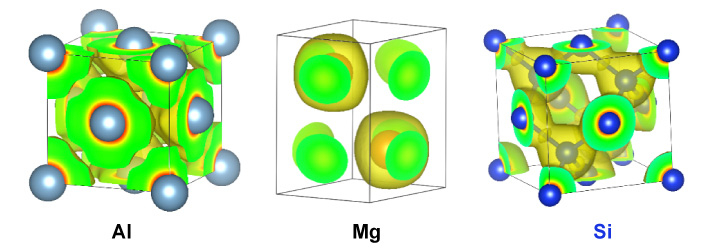
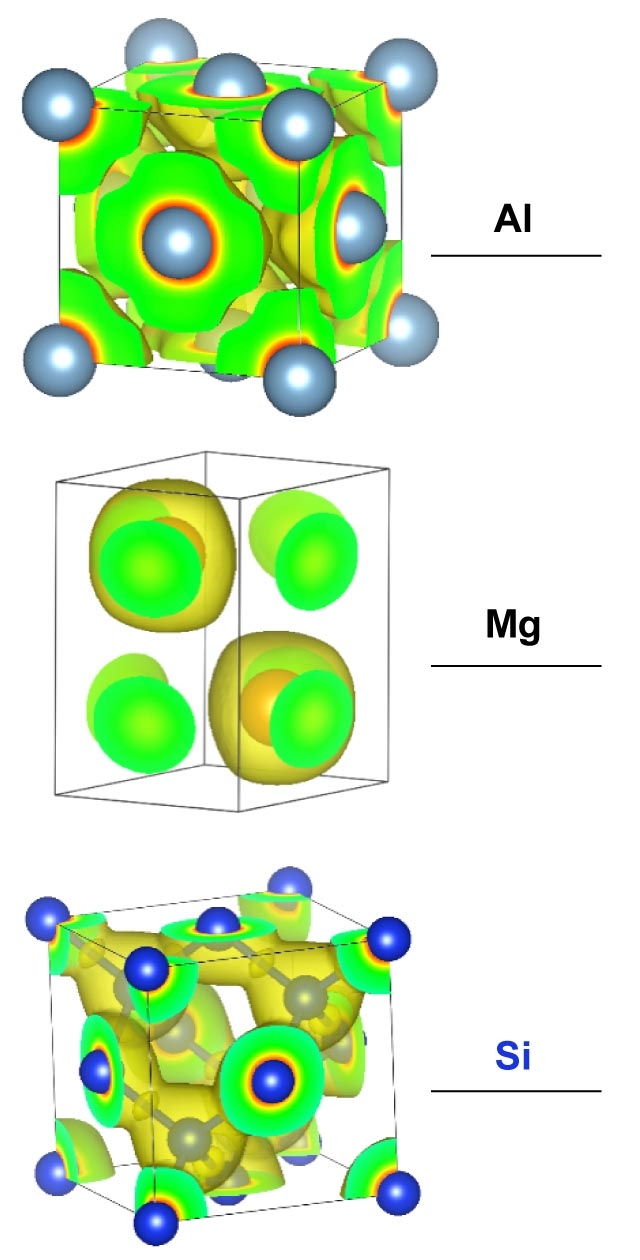
Kinetic energy densities of Al, Mg, and Si crystals, which Manzhos and his team model to develop large-scale DFT methods.
The importance of basic research and interdisciplinary research
"I hope our research can be useful in other ways too. Of course, providing new algorithms to the world has its uses, and obviously renewable energy technologies are increasingly important, but I think our efforts in quantum chemistry for basic materials research could help in far less obvious ways," said Manzhos. "For example, it might surprise you to know that, only relatively recently, did China, one of the world's industrial powerhouses, gain the expertise to make the tips for ballpoint pens. Something so humble you probably take it for granted, but the specific way the ball point is made required specific expertise in metallurgy and manufacturing techniques. Some key elements of understanding what happens inside a steel – which might sound like a boring material in the age of metaverses - were only acquired in the 21st century. My point is, sometimes remarkably basic research is needed to spawn new products and industries, even if they're conceptually simple, and it's often the case that this basic research is related to materials and phenomena important in daily life."
The reason the work of Manzhos and his team can be useful in so many different contexts, is because at its core it's highly interdisciplinary, combining many fields that have already been mentioned. Though not limited to quantum chemistry, an increasing number of research groups around the world are embracing the idea of interdisciplinary practice as a way to add coherence to otherwise disparate fields of study and encourage a more holistic approach to carrying out research.
"I don't think research should be siloed. This idea of breadth is important, as the challenges of today cannot be met if you only focus on minute details. We need to think in a wider context. The different areas I've worked in and continue to explore have given me the opportunity to build a career by keeping my interests broad, but also detailed where needs be, without being overly general or excessively narrow and specific," said Manzhos. "This way of carrying out research requires a certain agility and a lot of flexibility. But I have become very comfortable with that, and I actually think that such an environment can be rewarding and motivational, especially for students."
Strategy to help students discover their potential
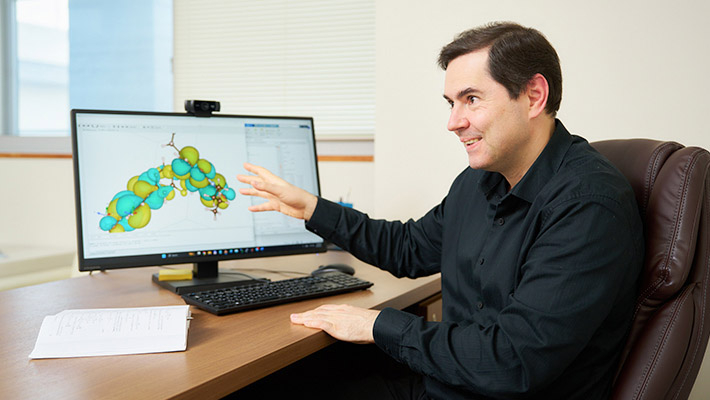
At Ihara-Manzhos Laboratory, Manzhos leads the group's materials modeling component, while Professor Manabu Ihara, also of the School of Materials and Chemical Technology, leads the experimental component, from materials to renewable energy systems. Their respective approaches work in synergy, while also offering newcomers a wide range of research opportunities. "In some ways, as our lab includes so many overlapping fields, I think it can be an easy sell to prospective students," Manzhos continued. "Students and early career researchers coming to our lab can easily find some area or another that suits them and their goals. In fact, by design, we encourage new starters to sort of bounce around the lab trying different things and exploring what's on offer. It's a strategy to help people discover their potential. Maybe they're into running experiments, or perhaps they prefer desk work, working on theoretical problems. Our lab can suit different personality types, which may be reflected in different ways of carrying out research. Essentially, students can join as generalists and become more specialized in time. I hope this direction inspires the next generation as much as it has my own."
Referencing the Environmental Energy Innovation Building, where Ihara-Manzhos Laboratory is housed, Manzhos concluded, "One thing's for sure. Our building is certainly a source for inspiration. It expresses some of our core values in its physical make up. By showing off solar panels, and integrating fuel cell technology, it helps solidify some of the more abstract ideas we might experience throughout the course of our studies. It's worth coming to see if you happen to be in the area."

The Environmental Energy Innovation Building, which Professor Manabu Ihara played a significant role in designing, is covered with 4,570 solar panels. Its smart energy system "Ene-Swallow" enables efficient operation of distributed power supply, and a unique power prediction model based on real-time data is used to perform peak-cut control.
1 Density Functional Theory (DFT)
Density Functional Theory (DFT) is a computational method used in physics and chemistry to understand the behavior of electrons in atoms and molecules. Instead of dealing with the complex wave functions of individual electrons, DFT looks at electron density, i.e., the distribution of electrons in space, to describe a system's total energy. DFT can provide valuable insights into the molecular structures, reaction energies, and electronic properties of materials.
2 Density Functional-Based Tight Binding (DFTB)
Density Functional-Based Tight Binding (DFTB) is a quantum mechanical method used for simulating the electronic structure and properties of materials. The tight-binding approach simplifies electronic structure calculations by approximating the electron-electron interactions. DFTB takes the method a step further by introducing electron density functional approximations, allowing for a more accurate description of the electronic structure compared to traditional tight-binding methods.

Associate Professor Sergei Manzhos
Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- 2021 to PresentAssociate Professor, Department of Chemical Science and Engineering, School of Materials and Chemical Technology
- 2019-2021Associate Professor, Centre Énergie Matériaux Télécommunications, Institut National de la Recherche Scientifique
- 2012-2019Assistant Professor, Department of Mechanical Engineering, National University of Singapore
- 2010-2012Project Assistant Professor, Research Center for Advanced Science and Technology (RCAST), University of Tokyo
- 2008-2010Project Assistant Professor, Chemical Systems Engineering, University of Tokyo
- 2005-2007Postdoctoral Fellow, Department of Chemistry, University of Montreal
- 2004Ph.D. (Chemistry), Queen's University
- 1999MSc (Radio Physics), Kharkiv National University
The Special Topics component of the Tokyo Tech Website shines a spotlight on recent developments in research and education, achievements of its community members, and special events and news from the Institute.
Past features can be viewed in the Special Topics Gallery.
. Any information published on this site will be valid in relation to Science Tokyo.















