Researchers at Tokyo Institute of Technology (Tokyo Tech) report that anthropogenic sources of carbonyl sulfide (OCS), not just oceanic sources, account for much of the missing source of OCS in the atmosphere. Their findings provide better context for estimates of global photosynthesis (taking up CO2) using OCS dynamics.
Image: A conceptual Japanese-style artwork depicting the sources and sinks of carbonyl sulfide
By using sulfur isotope distributions as new constraints on the atmospheric carbonyl sulfide budget, the study revealed that anthropogenic sources are likely to be more important than previously thought.
Image credit: Mindy Takamiya
Carbonyl sulfide (OCS) is the most stable and abundant sulfur-containing gas in the atmosphere. It is derived from both natural and anthropogenic sources and is of key interest to scientists investigating how much carbon dioxide (CO2) plants take out of the atmosphere for photosynthesis. Measuring CO2 alone cannot provide estimates of photosynthesis (taking up CO2) because plants also release CO2 through respiration. In contrast, OCS is taken up like CO2 but is not released by respiration, and can therefore provide valuable information about the rate of global photosynthesis.
Understanding the precise OCS budget (the balance of source and sink) is an ongoing challenge. The most critical point of uncertainty related to the OCS budget is its missing source. Lack of observational evidence has so far led to debate about whether the missing OCS source is oceanic or anthropogenic emission.
In a new study published in Proceedings of the National Academy of Sciences of the United states of America (PNAS), researchers from Tokyo Tech's School of Materials and Chemical Technology and Earth-Life Science Institute (ELSI) have used a unique method of measuring sulfur isotope ratios (minor 34S isotope abundance relative to major isotope 32S, 34S/32S) of OCS that enabled them to distinguish oceanic and anthropogenic OCS sources.
"It's very exciting that we were able to separate anthropogenic and oceanic signals for OCS sources based on sulfur isotope ratios," says Shohei Hattori, an assistant professor at Tokyo Tech and lead author of the study. "These measurements required at least 200 liters of air for each sample measurement. We overcame this challenge by developing a new sampling system, and eventually succeeded in measuring sulfur isotope ratios of the atmospheric OCS."
The team found a north–south latitudinal gradient in the 34S isotope abundance corresponding to OCS concentrations during wintertime in eastern Asia. Their results provide evidence of the importance of anthropogenic OCS emissions from China. Also, by using the sulfur isotope level of OCS as a new constraint, they found that anthropogenic OCS sources, and not only oceanic sources, are likely to be major constituents of the missing source of atmospheric OCS.
"The higher relevance of anthropogenic OCS at mid-to-low latitudes has implications for understanding climate change and stratospheric chemistry in both past and future contexts," says co-author Kazuki Kamezaki.
Given that the historical estimation of how much CO2 is taken up by plants is sensitive to the estimate of the anthropogenic OCS inventory, a more detailed picture of the OCS budget revealed by sulfur isotopic approach will enable more precise estimation of its interactions with global change. The research team will continue to undertake more observations to make detailed quantitative estimates and predictions of the global photosynthesis rate.
"Our sulfur isotopic approach for measuring atmospheric OCS is an important step, but more observations, together with analysis using a chemical transport model, will enable detailed quantitative conclusions," Hattori says.
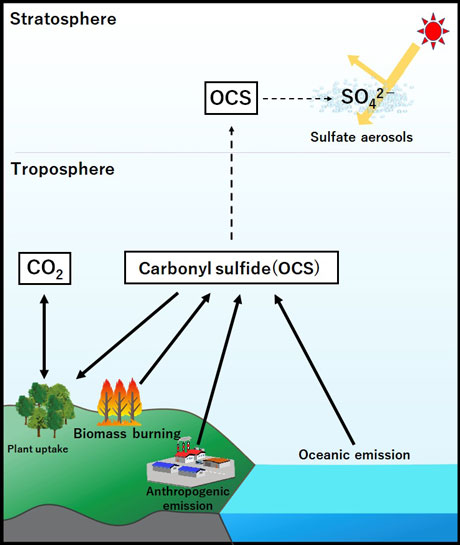
Figure 1. Scheme of the biogeochemical OCS cycle.
Carbonyl sulfide (OCS) in the atmosphere is derived from both oceanic and anthropogenic sources.
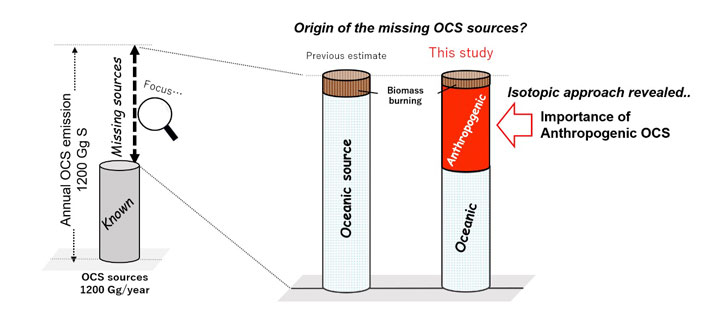
Figure 2. Tracking down missing OCS sources.
The team's sulfur isotopic approach revealed the importance of anthropogenic OCS emissions in the global OCS budget.
Reference
Authors : |
Hattori, S.a, Kamezaki, K.a and Yoshida, N.a,b |
Title of original paper : |
Constraining the atmospheric OCS budget from sulfur isotopes. |
Journal : |
PNAS |
DOI : |
|
Affiliations : |
a Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology
b Earth-Life Science Institute, Tokyo Institute of Technology
|
. Any information published on this site will be valid in relation to Science Tokyo.






