1 Fritz Haber and Carl Bosch
Haber and Bosch established the first method of ammonia synthesis that directly synthesized airborne hydrogen and nitrogen. This is often called the Haber-Bosch (HB) process. Haber received the Nobel Prize in Chemistry in 1918 for his research on ammonia synthesis research, and Bosch received the prize in 1931 for the high pressure reaction process he developed.
2 Catalyst
A substance which accelerates a chemical reaction or develops specific reactions. The catalyst itself does not change because of the reaction. It reduces the activation energy generated in chemical reactions, but cannot promote reactions that are not naturally generated.
3 Stoichiometry
The theory of the relative proportion of reactants and products in a chemical reaction. If X amount of an elementary reaction are required to complete one overall reaction, then X is the stoichiometric number of that elementary reaction. X is also the stoichiometric number of the elementary reaction that is the rate-determining step of the overall chemical reaction.
4 Rate-determining step
The slowest step of a chemical reaction that determines the speed at which the overall reaction proceeds.
5 Isotopes
Variants of a chemical element that differ in neutron number. The isotope effect is the variation in the physical properties and reactivity of isotopes or isotopic ratios, which is caused by isotopes of a given element making up substances and compounds.
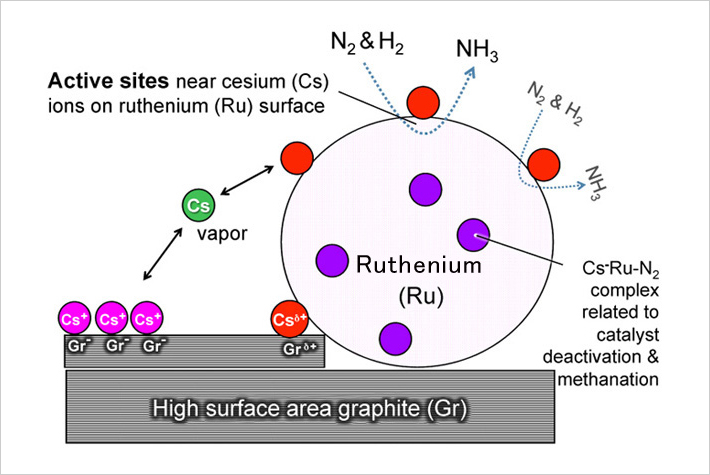
6 Ruthenium
A rare platinum-group metal also classified as a precious metal. Ruthenium-osmium alloys are used for fountain pen nibs. Often used as a catalyst to hydrogenate unsaturated bonds in organic chemistry.
7 C12A7 (12CaO・7Al2O3)
A crystal structure with a cage-like skeleton that is 0.5-nm in diameter. In 2003, Hosono and his team discovered that inserting electrons in the cages forms stable electrides (ionic compounds in which electrons serve as anions). It was clarified that a C12A7 electride conducts electricity as a metal, and is superconductive at low temperatures.
. Any information published on this site will be valid in relation to Science Tokyo.