Research
Research
FACES: Tokyo Tech Researchers, Issue 41
Issue 41
Masao Takeyama
Professor, Department of Materials Science and Engineering, School of Materials and Chemical Technology
At the School of Materials and Chemical Technology, Professor Masao Takeyama studies the microstructure of metals for use under high temperatures and pressures, as well as mechanisms for increasing their performance.
Iron is an abundant resource that is inexpensive and has played a key role in human civilization. Takeyama enthusiastically stated, "iron is a gift from God to humankind." He continued, "Iron is an amazing substance with many unusual features. Unlike ordinary metals, as the temperature increases, the crystal structure changes from a body-centered cubic (bcc) crystal called ferrite to a denser face-centered cubic (fcc) crystal called austenite. By utilizing this change in crystal structure (α-γ phase transformation1), it's possible to induce different properties. This makes it a very attractive material because it can be used in various applications, including infrastructure. Iron still has much potential."
Takeyama is working to uncover such untapped potential in metals, particularly in iron and the possibility of its use as a high-temperature material.
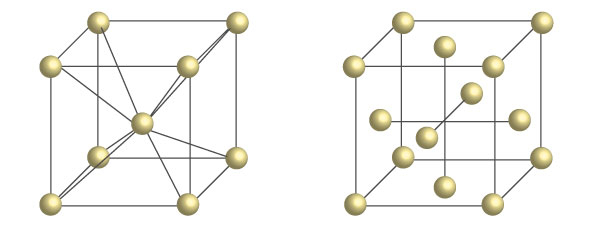 Body-centered cubic (bcc) structure of ferrite (left), and
Body-centered cubic (bcc) structure of ferrite (left), and
face-centered cubic (fcc) structure of austenite (right).
Takeyama's research is founded upon the Phase Diagram-based Microstructure Design Principle. It is a principle for controlling the microstructure of metals requiring high heat resistance and strength at the atomic level in extremely harsh environments, such as at power plants and in aircraft engines.
"There is a widespread belief that the thermal power generation sector, which uses fossil fuels and accounts for about 30% of Japan's CO2 emissions, should move toward carbon neutrality. But wind power and solar power alone are not enough to cover Japan's total annual electricity usage (approximately 1 trillion kWh). It is the responsibility of researchers to have discussions based on accurate data rather than ideals. In consideration of a stable energy supply, I think that fuels will change to carbon-free fuels such as hydrogen and ammonia, but these are still thermal power. It is believed that thermal power generation will play an important role in the future. And if that happens, metallic materials that can be used in harsh environments at increasingly high temperatures and pressures will be needed. My goal is to find the principles that can serve as the basis for the design and development of heat-resistant materials, so it will definitely help to realize a carbon-free society. Japan's research on heat-resistant materials is the most advanced in the world. Accelerating this research and preparing properly as we move toward a carbon-free society is key."

"As a researcher, I first want to understand the principles of metals. Then, based on scientific principles, I can suggest to the industry, ‘This is a good way to improve the performance of a metal.' In this way, I hope to support innovation through industry-academia collaboration and contribute, from a materials standpoint, to resolving the energy and environmental problems facing the world. I think that is my role."
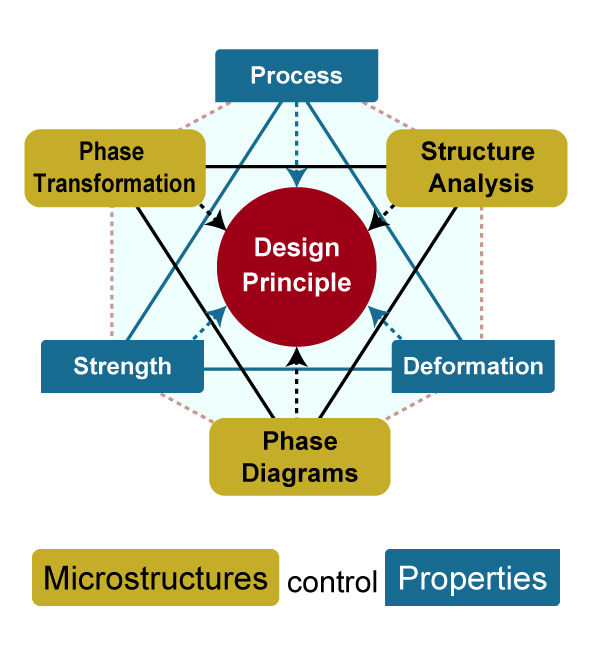 Hexagon of Disciplines for Design Principle
Hexagon of Disciplines for Design Principle
Takeyama's research is based on the idea that properties are determined by the microstructure design. He first tries to understand the required properties and then designs the microstructure at the atomic level. "Many people think that the properties of metals and alloys are determined by the composition of the alloys. However, even if the composition remains the same, the performance changes drastically when the microstructure is changed. That is the real pleasure of material design," according to Takeyama.
The "equilibrium phase diagram" is a guideline for microstructure design. A phase diagram of a metal is a map that shows what kind of phases exist when a certain alloy is kept at a certain temperature for an infinite time. "When designing new high-temperature materials, phase diagrams are the most important means of achieving the ultimate goal. However, many existing multi-component phase diagrams are not reliable, and many systems have not been studied. For ones that have not been studied, I make them myself through experiments and calculations. This is one of the research subjects that not so many researchers want to do because it takes a lot of time and effort," according to Takeyama. Materials designed in this way are prototyped, analyzed, and verified using many experimental instruments in the laboratory. Using multiple engineering approaches, he has created new materials that perform as required.

Analyzing the elements contained in samples (alloys).
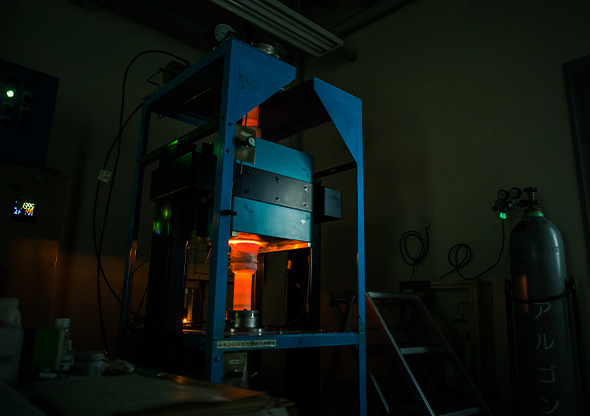
Controlling the microstructures of a metallic materials in a high temperature environment.
A keyword of Takeyama's research is "intermetallic compounds." An intermetallic compound is a substance that gains new properties when two or more types of metallic elements are bonded.
First, let's consider research on materials used for power plants. With thermal power generation there are "gas turbines" that use liquefied natural gas as fuel and steam turbines that use coal as a heat source. The former consists of a compressor that compresses a large amount of intaken air, a combustor that burns the compressed air and fuel, and a turbine that uses the high-temperature, high-pressure combustion gas to rotate blades, and the rotational force of the turbine is used to rotate the generator to produce electricity. A jet engine in an aircraft uses this mechanism, and the rotational force of the turbine is used for propulsion. On the other hand, a steam turbine consists of a boiler (heat exchanger) that converts water into high-temperature, high-pressure steam (supercritical state) by burning fossil fuel, and a turbine that rotates blades using this steam, and as with a gas turbine, the rotational force is used to generate electricity. There is also a highly efficient combined power system where the exhaust heat of a gas turbine is used to rotate a steam turbine, and in both cases, efficiency improves as the temperature of the heat medium rotating the blades increases.
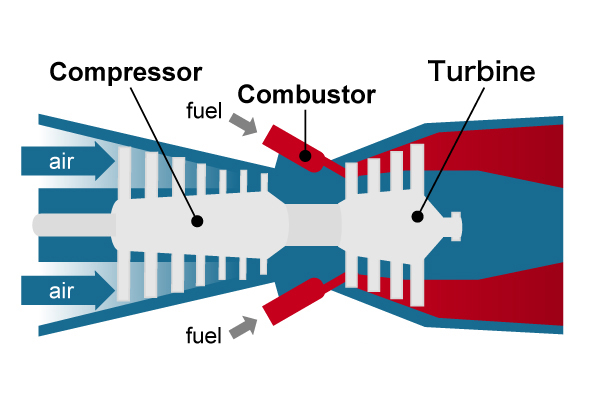
Principle of gas turbine system
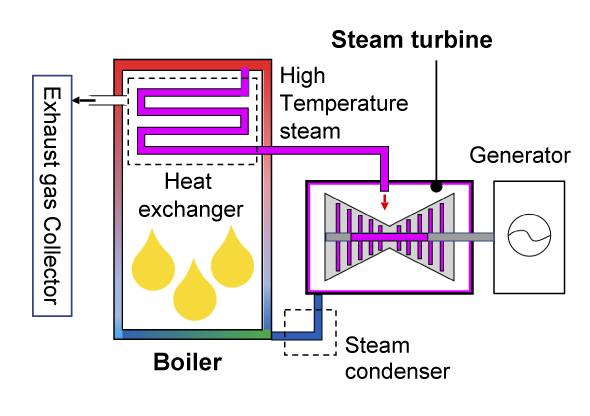
Principle of Steam turbine system
The combustion gas temperature of gas turbines reaches at least 1,600℃, and nickel (Ni)-based superalloys are used for its components. For steam turbines, the steam temperature is about 600℃, and the heat exchanger of the boiler, the piping that guides the steam to the turbine chamber, and members including the turbine chamber are usually made of an iron (ferrite)-based alloy2 with a bcc structure. Power generated with a steam temperature of about 600℃ is called ultra-supercritical (USC) power generation, and the world's highest power generation efficiency is achieved using Japan's material technologies. However, in recent years, to reduce CO2 gas emissions and maintain a stable energy supply, development of advanced ultra-supercritical (A-USC) power plants has been promoted in Japan, Europe, and the United States, where the steam temperature for thermal power generation is raised to 700℃ or higher in order to further improve power generation efficiency. Normal ferrite-based alloys (where use is limit to about 650℃) cannot be used for these. In other words, new high-strength, heat-resistant metallic materials need to be developed for such A-USC plants. Since such power plants operate continuously every day, the material must not fail even when exposed to high temperatures for 100,000 hours (10 years) or more while experiencing a stress of 100 MPa at the operating temperature. This means the material must have excellent creep strength. Creep is a phenomenon in which strain increases over time when stress is applied continuously to an object at high temperature. Normally, at such temperatures, Ni-based alloys that have a proven track record as jet engine materials are the candidate materials. The goal of Takeyama's research is to realize materials made from iron-based alloys that excel in both heat resistance and creep rupture strength.
"It is a dream to have an iron-based alloy with a creep rupture strength of 100 MPa or more for 100,000 hours at 800℃. Normally, only Ni-based alloys are considered. However, nickel is an expensive rare metal. The price is about 50 times higher than that of iron, and it's increasing. Our goal was to see how high of a temperature we can achieve with iron. This is extremely ambitious and challenging. We have worked on achieving this by using iron-based alloys, which is an abundant resource and is inexpensive," says Takeyama.
Takeyama's attempt was successful. "What makes iron interesting is that the crystal structure changes greatly depending on the temperature and the type and amount of elements that are added." Takeyama was focusing on the crystal structure of iron at high temperatures. Iron has the same fcc structure (austenite) as Ni-based alloys at high temperatures. "Various heat-resistant materials based on austenite have already been developed, but their heat-resistance performance is low because carbides (compounds of carbon and metal elements) are used for the strengthening phase. However, Ni-based alloys have excellent heat resistance because thermodynamically stable intermetallic compounds are used for the strengthening phase. That is why the creep strength of austenite is inferior to that of Ni-based alloys even though they have the same fcc structure. If an intermetallic compound is used for the strengthening phase and the grain boundaries, which becomes a weakening factor at high temperatures, are covered with the intermetallic compound phases, and the heat resistance can be increased to 700℃ or higher," according to Takeyama. "I had a nagging question for many years as to why there are no austenitic heat-resistant materials that use intermetallic compounds in the strengthening phase," he recalled.
Therefore, Takeyama asked many different people why there are no austenitic heat-resistant materials that use intermetallic compounds. He found that, while it is was assumed that intermetallic compounds are hard and brittle and cannot be used, no one had actually tried to use them. The compounds have been intentionally avoided for designing alloys. "It is important to question common beliefs. I carefully examined the ternary phase diagram of Fe-Ni-M (M is a transition metal element).
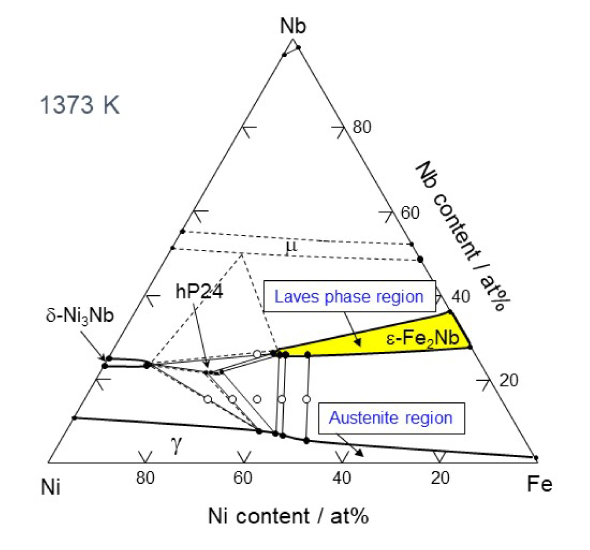
Experimentally determined Fe-Ni-Nb ternary phase diagram
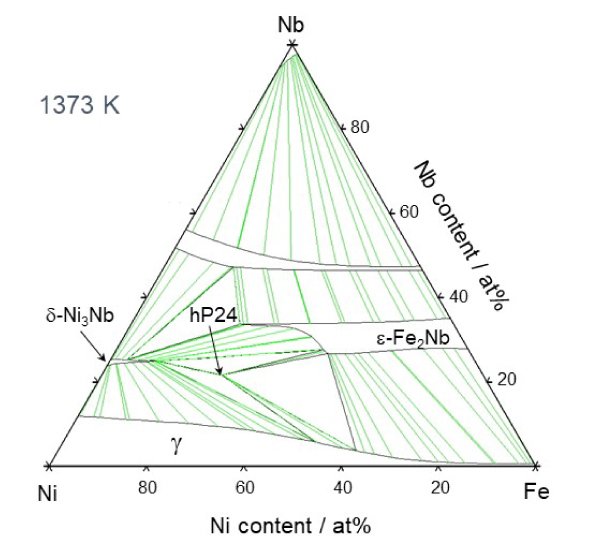
Calculated Fe-Ni-Nb ternary phase diagram
Based on this, I was able to identify a region where an Fe2M type intermetallic compound phase consisting of two iron atoms and one M element, called a Laves phase3, exists. Laves phases had been found in materials that ruptured when exposed to high temperatures for a long period of time, so this phase was considered to be harmful. Therefore, materials have been designed to avoid formation of Laves phases. However, there is no consideration of time axis in the thought. Laves phases are stable phases that form after a long period of time at high temperature. The cause of the deterioration is actually not the Laves phases, but the microstructural change and instability of the carbide that was first used for the strengthening phase, which changes to an intermetallic compound over time. Therefore, I believed that if Laves phases were used for the strengthening phase from the beginning, it would be an effective strengthening phase for improving high-temperature creep strength."
Takeyama began researching austenitic heat-resistant materials that utilize Laves phases. First, he established guiding principles for microstructure design for that purpose and devised a new strengthening method called "grain boundary precipitation strengthening (GBPS)." In this mechanism, the grain boundaries, which are the interfaces between crystal grains, are decorated with Laves phases, and the higher the degree of decoration called "area fraction ρ", the higher the creep strength.
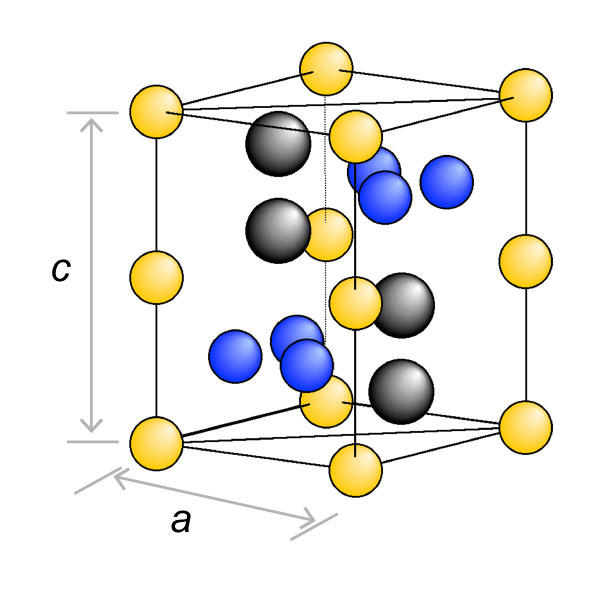 Crystal structure of Laves phase
Crystal structure of Laves phase
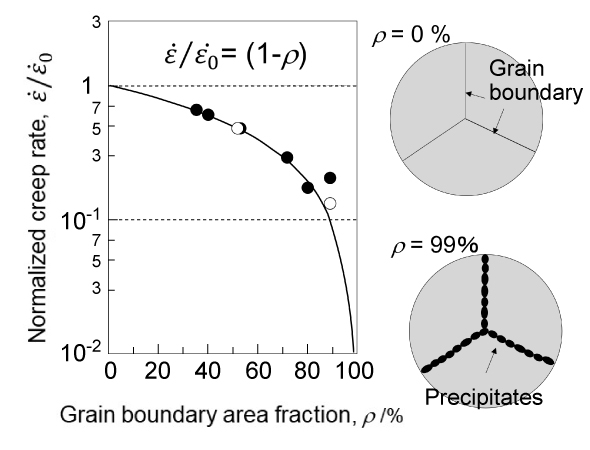 Grain Boundary Precipitation Strengthening Mechanism
Grain Boundary Precipitation Strengthening Mechanism
As he expected, the iron-based alloy Takeyama designed was found to have a creep strength comparable to that of nickel-based alloys. He succeeded in demonstrating that even Fe-based alloys can obtain creep strength comparable to that of Ni-based alloys.
"The idea of GBPS actually dates back to when I was a graduate student. In my dissertation, I investigated the relationship between grain boundary coverage and creep strength due to precipitates4. I noticed that the higher the grain boundary coverage between the grains, the higher the creep strength."

Takeyama also designs intermetallic materials for aircraft engines.
The structure of a jet engine is basically the same as that of a gas turbine for power generation used on the ground, but the decisive difference is that, since it is a flying object, weight must be minimized. To improve fuel efficiency, it is important to increase the compression ratio of the air taken in and to use the energy from combustion as effectively as possible. The temperature of the compressed air is about 600℃, but recently there has been a demand for stronger materials that can withstand 700℃ with a higher compression ratio. A turbine is divided into two parts: a high-pressure turbines (HPT), into which combustion gas is blown at 1,000℃ or higher, and low-pressure turbines (LPT), which further utilize this gas to generate power.

Until now, Ni-based alloys have been used for the compressor blades and LPT blades of jet engines. However, to improve fuel efficiency, materials that are lighter and with higher strength are needed. Takeyama is studying titanium aluminide (TiAl)-based alloys as such a material. Titanium aluminide has a specific gravity that is about half that of nickel, and has excellent high-temperature strength and oxidation resistance. As an intermetallic compound material, it is attracting the most attention as a material for moving blades.
"LPT blades manufactured with the TiAl-based alloys through casting5 have been installed in Boeing 787 engines since 2012, and it took about 20 years to put these into practical use. In order to put a new material into practical use as an engine material, which can impact human life, it must pass various safety performance tests from the manufacturing process to the strength. In 2016, LPT blades formed by forging6 were installed in Airbus A320neo engines. Forged materials have better fracture toughness compared to cast materials. I established the world's first guiding principles for microstructure design through this forging process. Researchers and companies in Europe quickly adopted this," Takeyama explained.
The approach for establishing these design guidance principles is the same as for the Fe-based alloys development strengthened by Laves phase as mentioned above.
"First, I made a ternary phase diagram of Ti-Al-M (M is a transition metal element) where another element is added to the Ti and Al. There was no information for the ternary phase diagrams at that time, so we made it ourselves. We focused on the equilibrium phases at high temperature with the TiAl phase (called the γ phase), which is the basis of the alloy. From this, we found a Ti3Al phase (called α2 phase) and Ti phase with a bcc structure (called β phase) as equilibrium phases with the γ phase. There are regions where these three phases coexist and the composition of the coexisting region greatly depends on the temperature. From this, we found a unique phase transformation that does not exist in the Ti-Al binary system. Using this phase transformation, a forging process became possible, which was believed to be impossible until then. I established microstructure and process design principles for wrought TiAl-based alloys with high-strength and high-toughness. This research report was made in the latter half of the 1990s I believe, but at that time, no one understood it and it received little attention. At that time, casting was mainstream and it was believed that forging brittle intermetallic compounds was impossible. However, some people took notice. These were European universities and companies, which led to its practical application." says Takeyama.
Takeyama demonstrated that "the microstructure determines the characteristics" in his research on titanium aluminide, and he clarified the mechanism for increasing the strength. To further improve the characteristics, he has recently been working on four-dimensional microstructure analysis that incorporates a time axis into the three-dimensional analysis.
Here, the surface of a metal material was thinly sliced every 20 nm and photographed, and this was repeated 500 times or more. These images were then integrated into a three-dimensional image of the TiAl-based alloy. It was found that the white phase, which only looked like particles in the two-dimensional image, actually had an elongated plate-like shape, which is what helps improve strength and toughness.
Some of Takeyama's research on iron-based alloys was conducted under the Japan Science and Technology Agency's (JST) "Advanced Low Carbon Technology Research and Development Program (ALCA)," and research on titanium aluminide was conducted with support from the Cabinet Office's Cross-ministerial Strategic Innovation Promotion Program (SIP) "Structural Materials for Innovation," with Takeyama taking the lead on research for these projects. This achievement was highly regarded, and Takeyama received the Academic Achievement Award from the Iron and Steel Institute of Japan in 2018 and the Honda Memorial Lecture Award from the Japan Institute of Metals and Materials in 2020.
Takeyama first decided to be a metallurgist when he was in high school. "In high school, I once asked my father, who was a professor at Hokkaido University researching metal materials, what he was researching. He said, 'Why iron becomes harder when carbon is added.' I then asked, 'is that research interesting?' He answered, 'It's very interesting.' That is why I decided to follow the same path."
Takeyama has the following message to young people considering careers in research. "Be full of curiosity, have questions, make effort, and be humble. I've been researching titanium aluminide for 30 years. I've probably studied this material longer than anyone in the world. I think perseverance is power. I hope that you will pursue themes that you really find interesting, and not just themes that make it easy to secure funding or to write papers according to trends. Also, do not be deterred by common beliefs or prejudices. Instead of just accepting what is written in newspapers and textbooks, or what a teacher says as true, you should ask 'Is that really true?' Never stop being curious and wanting to make sure for yourself."

"Phase" refers to the state of matter such as gas, liquid, or solid. Phase transformation refers to a change in the crystal structure of a material. As a simple example, water (liquid phase) turns into ice (solid phase) below 0℃ and vapor (gas phase) at 100℃. There are also solid phase transformations. Iron is a bcc-structured iron (α phase) at low temperature to 910℃, but undergoes a phase transformation to fcc-structured iron (γ phase) at 910℃ to 1400℃.
An alloy containing at least 50% iron is called an iron-based alloy. Similarly, with nickel, it is called a nickel-based alloy, and with titanium aluminide, it is called a titanium-aluminide-based alloy.
Phases of intermetallic compounds with high electrical conductivity but that are characterized by extremely low plasticity and ductility, which are a characteristic of metals.
A product material where an excessively dissolved atom is separated from a parent crystal and is newly formed.
A processing method in which a material is heated to a temperature higher than the melting point to make it a liquid, and it is then poured into a mold to be cooled and hardened.
A processing method that increases strength by hammering the metal and applying pressure to form it into a desired shape.

Masao Takeyama
Professor, School of Materials and Chemical Technology, Department of Materials Science and Engineering
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016
The Special Topics component of the Tokyo Tech Website shines a spotlight on recent developments in research and education, achievements of its community members, and special events and news from the Institute.
Past features can be viewed in the Special Topics Gallery.
Published: September 2021